Monographs: Dosage forms: Specific monographs: Melarsoprol injection (Melarsoproli injectio)
Description. Melarsoprol injection is a clear, colourless to almost colourless solution.
Category. Antitrypanosomal drug.
Storage. Melarsoprol injection should be protected from light.
Additional information. Strength in the current WHO Model list of essential medicines (EML): 3.6%.
Melarsoprol (formula: C12H15AsN6OS2) has a relative molecular mass of 398.3 and the chemical name: 2-[4-(4,6-Diamino-1,3,5-triazin-2-ylamino)phenyl]-1,3,2-dithiarsolan-4-yl-methanol; CAS Reg. No. 494-79-1.
Requirements
Complies with the monograph for Parenteral preparations.
Definition. Melarsoprol injection is a sterile solution of melarsoprol in propylene glycol, containing 5% of water. The injection is normally prepared by heating equimolar amounts of melarsen oxide and dimercaprol in propylene glycol and adding water. The solution is sterilized by "Heating in an autoclave" or by any other suitable method (see 5.8 Methods of sterilization).
Melarsoprol injection contains not less than 3.4% and not more than 3.8% of C12H15AsN6OS2.
Identity tests
A. To a volume of the injection equivalent to 35 mg of Melarsoprol, add 5 mL of dilute hypophosphorous acid TS and heat on a water-bath; a yellow precipitate is produced.
B. To a similar volume of the injection as for test A add 4 mL of sodium hydroxide (~300 g/L) TS and heat to boiling; the evolved vapours turn red litmus paper R to blue.
C. To a further volume of the injection as for test A add 2 mL of water, 1 mL of ammonia (~260 g/L) TS and 3 mL of silver nitrate (40 g/L) TS; a yellow precipitate is produced which is soluble in nitric acid (~1000 g/L) TS.
Relative density. 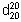 = 1.050 –1.056.
= 1.050 –1.056.
Inorganic arsenic. To a volume of the injection equivalent to 0.18 g of Melarsoprol add 4 mL of sodium hydroxide (1 mol/L) VS, shake and add 3 mL of sulfuric acid (~190 g/L) TS; a precipitate is produced. Shake, dilute to 50 mL with water and mix. Allow to stand for 2–3 minutes, filter and dilute 10 mL of the clear filtrate to 100 mL with water. Use 30 mL of this solution and proceed as described under 2.2.5 Limit test for arsenic; the arsenic content is not more than 30 mg/g.
Clarity and colour of solution. The injection is clear and not more intensely coloured than standard colour solution Yw3 when compared as described under 1.11.1 Colour of liquids.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, method A, using 0.5 g of the injection; the water content is not less than 40 mg/g and not more than 60 mg/g.
Assay. Transfer an accurately measured volume of the injection equivalent to about 0.18 g of Melarsoprol to a long-necked, 200 mL flask. While cooling add a mixture of 12 mL of sulfuric acid (~1760 g/L) TS and 40 mL of hydrogen peroxide (~330 g/L) TS. Heat gently and carefully until white vapours appear in the neck of the flask (heating time: 45–60 minutes). Cool slightly, add 8 g of potassium sulfate R and 0.05 g of starch R and heat to boiling until decolorization occurs (heating time: 60–90 minutes). Cool and add slowly 70 mL of water. Transfer to a conical flask, rinse with 50 mL of water, add 0.05 mL of phenolphthalein/ethanol TS, then add slowly while cooling 30–40 mL of sodium hydroxide (~300 g/L) TS until a light pink colour is obtained. Add, drop by drop, sulfuric acid (~190 g/L) TS until decolorized, then add 3 g of sodium hydrogen carbonate R and 50 mL of water. Titrate with iodine (0.05 mol/L) VS.
Each mL of iodine (0.05 mol/L) VS is equivalent to 19.92 mg of C12H15AsN6OS2.
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains less than 1.39 IU of endotoxin per mg Melarsoprol.