Monographs: Dosage forms: Specific monographs: Metronidazole injection (Metronidazoli injectio)
Description. Metronidazole injection is a colourless solution.
Category. Antibacterial drug; antiamoebic drug.
Storage. Metronidazole should be kept in a single-dose container, protected from light.
Additional information. Strength in the current WHO Model list of essential medicines: 5 mg/mL.
Requirements
Comply with the monograph for "Parenteral preparations".
Definition. Metronidazole injection is a sterile solution of metronidazole in water for injections. The solution is sterilized by a suitable method (see 5.8 Methods of sterilization).
Metronidazole injection contains not less than 90.0% and not more than 110.0% of the amount of C6H9N3O3 stated on the label.
Identity tests
A. Dilute a volume of the injection equivalent to 20 mg of Metronidazole to 100 mL with a mixture of solvents composed of 1 mL of sulfuric acid (~1760 g/l) TS in 350 mL of methanol R. Further dilute 1 mL of this solution to 10 mL using the same mixture of solvents. The absorption spectrum, when observed between 220 nm and 350 nm, is qualitatively similar to that of a 20 μg/mL solution of metronidazole RS in the same mixture of solvents.
B. To a volume of the injection equivalent to 5 mg of Metronidazole, add 0.05 g of 4-dimethylaminobenzaldehyde R dissolved in 2 mL of hydrochloric acid (~70 g/l) TS; a yellowish colour is produced. Add 0.05 g of zinc R powder; the colour changes to red-orange.
pH value. pH of the injection, 4.5-7.0.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R4 as the coating substance and acetone R as the mobile phase. Apply separately to the plate 10 μl of each of the following two solutions. For solution (A) evaporate a volume of the injection equivalent to 0.05 g of Metronidazole to dryness on a water-bath and dissolve the residue in 5 mL of a mixture of equal volumes of methanol R and chloroform R. For solution (B) dissolve 20 mg of 2-methyl-5-nitroimidazole R in 10 mL of the same mixture of solvents. After removing the plate from the chromatographic chamber, allow it to dry in air, and examine the chromatogram in ultraviolet light (254 nm).
The spot obtained with solution B is more intense than any corresponding spot obtained with solution A.
Assay. Transfer an accurately measured volume of the solution equivalent to about 10 mg of Metronidazole to a 100-mL volumetric flask. Add 80 mL of hydrochloric acid (0.1 mol/l) VS and shake. Dilute to volume with the same acid and mix well. Filter through a dry filter-paper, discarding the first few mL of the filtrate. Dilute accurately 5.0 mL of the filtrate to 200 mL with hydrochloric acid (0.1 mol/l) VS and mix well.
Measure the absorbance of the resulting solution in a 1-cm layer at the maximum at about 277 nm. Calculate the content of C6H9N3O3, using the absorptivity value of 37.7 (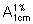 = 377).
= 377).
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins. Dilute the injection, if necessary, with water LAL to give a solution containing 5 mg per mL (solution A). Solution A contains not more than 3.5 IU of endotoxin per mL. Carry out the test using the maximum valid dilution of solution A calculated from the declared sensitivity of the lysate used in the test.