Methods of Analysis: 1. Physical and Physicochemical Methods: 1.14.3 Column chromatography
In adsorption column chromatography the solid support (e.g. activated alumina, powdered cellulose, silicic acid, or kieselguhr) is packed as a dry solid or as a slurry into a tube (glass, plastic, or other suitable material) having a restricted orifice (usually protected by a sintered glass disc) for efflux of the mobile phase. A solution of the material to be subjected to chromatography is added to the top of the column and allowed to flow into the adsorbent; the solvent that constitutes the mobile phase is then introduced in the top of the column and allowed to flow downwards either by gravity or by application of positive pressure; during this procedure care should be taken to ensure that the top of the column does not become dry. The effluent solution (known as the eluate) is monitored in either a continuous fashion (for example, with a flow-through ultraviolet absorption cell) or a stepwise fashion (for example, by collection of fractions at intervals determined by time or by volume or weight of eluate and subsequent examination of each fraction for the separated component). The need to examine individually many fractions in order to obtain a completely quantitative assessment of a substance has resulted in a decline in the use of classical column chromatographic procedures in recent years; where they do continue to be used, there is a natural tendency to favour methods of detection and determination that are readily adaptable to automated processes.
In partition column chromatography, a liquid stationary phase, which should be substantially immiscible with the mobile phase, is adsorbed to the surface of the solid adsorbent. Chromatography is carried out as described for adsorption column chromatography, the mobile phase being saturated with the stationary phase before it is used for elution. Usually the solid adsorbent in partition chromatography is polar and the adsorbed stationary phase is also polar with respect to the mobile phase. The most widely used adsorbent in this connexion is a siliceous earth having an appropriate particle size to permit ready flow of the mobile phase. In certain cases reverse-phase partition chromatography is useful; in this case the polar adsorbent is rendered non-polar by silanizing or other means, such as treatment with paraffins, and the adsorbed stationary phase is less polar than the mobile phase.
In these partition systems the degree of partition of a compound is governed by its distribution coefficient between the two liquid phases and, in the case of compounds that dissociate, by the pH of the more polar of the two phases. Selective elution of components of a mixture can often be achieved by successive changes in the mobile phase or by changing the pH of the stationary phase by using a mobile phase consisting of a solution of an appropriate acid or base in an organic solvent.
Ion-exchange chromatography may be considered a case of chromatography where the solid phase contains an ion-exchange material, usually called ion-exchange resin.
Ion-exchange is defined as the reversible interchange between the ion present in the solution and the counter-ion of the resinous polymer, modified cellulose, or bonded silica gel support; it may be exemplified for the H+/Na+ exchange of a strongly acidic cation-exchange resin as:
RSO3H + Na+ 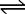 RSO3Na + H+
RSO3Na + H+
and for a Cl-/OH- strongly basic anion-exchange resin as:
R'N(CH3)3OH + Cl-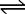 R'N(CH3)3Cl + OH-
R'N(CH3)3Cl + OH-
The selection of strong or weak resins, of either type, will largely depend on the pH at which the exchange is to be carried out and on the types of cation or anion that are to be exchanged. However, the strongly acidic and basic exchange resins will serve in most analytical applications. Their specific capacity may vary from 2 to 5 millimoles per gram (dry basis). In practice, a large (200-300%) excess of resin is used over the calculated stoichiometric requirement.
The laws governing the exchange reaction are complex, being in part described by mass action, ionic charge, and activity relationships. The selectivity coefficient is used to indicate the preference of the ion-exchange resin for the uptake of 2 (or more) ions from solution. Generally speaking, the resin will take up divalent (or higher) ions in preference to monovalent ions, and in the case of a choice between ions of the same valence, the resin will take up the heavier ion preferentially.
Treatment of the ion-exchange resin and preparation of the column. Usually the ion-exchange resin is immersed in water and allowed to swell for 24 hours; it is then packed into a suitable column and, in the case of an anion-exchange resin, converted to the basic form by passing sodium hydroxide (~80 g/l) TS through the column at a rate of about 3 mL per minute until the effluent is free of chloride, followed by carbon-dioxide-free water, R to remove alkalinity. In the case of a cation-exchange resin, conversion to the acidic form is achieved by passing hydrochloric acid (~70 g/l) TS through the column, followed by carbon-dioxide-free water R until the washings are neutral.
The prepared column is used in a similar manner to that described for adsorption column chromatography except that there is usually no need to monitor the effluent; according to the type of resin chosen and the type of material being determined the volume of effluent detailed in the particular application is collected and titrated with acid or base as appropriate, using a suitable indicator.
After the determination has been completed, the ion-exchange column may be regenerated by washing either with sodium hydroxide (~80 g/l) TS, for an anion-exchange column, or hydrochloric acid (~70 g/l) TS, for a cation-exchange column, followed by water until a neutral reaction is obtained.