Methods of Analysis: 1. Physical and Physicochemical Methods: 1.3 Determination of mass density, relative density and weight per millilitre
2017-01
The mass density (ρ) of a substance is the mass of one unit volume of the substance. In terms of SI base units, mass density is expressed in kilograms per cubic metre. However, in the International Pharmacopoeia the mass density is expressed in kilograms per litre (which is equivalent to grams per millilitre) at a temperature of 20 °C (ρ20) and it is corrected for buoyancy (i.e. reduced to vacuum conditions). For pharmacopoeial purposes the mass density of liquids is not measured directly but calculated from their relative density.
The relative density 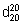 is the ratio of the mass of the substance in air at 20 °C to that of an equal volume of water at the same temperature. The term "relative density"
is the ratio of the mass of the substance in air at 20 °C to that of an equal volume of water at the same temperature. The term "relative density" 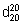 is equivalent to the formerly used term "specific gravity determined at 20 °C".
is equivalent to the formerly used term "specific gravity determined at 20 °C".
The relative density 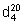 denotes the ratio of the mass of the substance in air at 20 °C to that of an equal volume of water at 4 °C. As the relative density of water at 20 °C is 0.998234, these values are related by the following equation:
denotes the ratio of the mass of the substance in air at 20 °C to that of an equal volume of water at 4 °C. As the relative density of water at 20 °C is 0.998234, these values are related by the following equation:
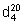 = 0.998234
= 0.998234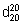
Recommended procedure
Determine the relative density (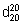 ) using a hydrostatic balance (only when the precision indicated in the monograph is three decimal digits) or a pycnometer.
) using a hydrostatic balance (only when the precision indicated in the monograph is three decimal digits) or a pycnometer.
If the value of mass density ρ20 (in kg/l or g/mL) is referred to in the monograph, carry out the measurement of relative density and from the value obtained calculate the mass density according to the formula:
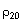 = 0.99703
= 0.99703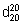 + 0.0012
+ 0.0012
Use of a hydrostatic balance
Use an instrument of suitable construction placed on a horizontal support. The plummet (diver) should be suspended on a thin wire, made preferably of platinum. To calibrate the instrument equilibrate the plummet in the air, then immerse it in the cylinder filled with water and equilibrate again by placing suitable riders (weights) at appropriate notches along the beam. The plummet should swim freely in the liquid. Fill the cylinder with the test liquid and carry out the measurement in a similar way. Take care that the length of the immersed portion of the suspending wire is similar in all measurements. The weight that has to be added to obtain the equilibrium in the test liquid (or to be subtracted in the case of liquids of density lower than that of water) gives directly the measure of its relative density.
Use of a pycnometer
Use a pycnometer of suitable form of a capacity of not less than 5 mL. Weigh accurately the empty, dry pycnometer, and fill it with the test liquid brought previously to a temperature of about 20 °C. Hold the filled pycnometer at a temperature of 20 ± 1 °C for about 30 minutes, adjust the liquid to the mark using, if necessary, a small strip of filter-paper to remove the excess and to wipe the inlet from the inside, and weigh accurately. Calculate the weight of the liquid in the pycnometer. Remove the liquid, clean and dry the pycnometer, repeat the measurement with carbon-dioxide-free water R, also at 20 ± 1 °C, and calculate the weight of water in the pycnometer. The ratio of the weights of the test liquid and of water gives the relative density (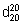 ).
).