Methods of Analysis: 2. Chemical methods: 2.5 Complexometric titrations
Complexing agents of value as titrants are aminopolycarboxylic acids that possess the characteristic group
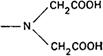
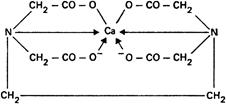
Such compounds are capable of forming chelate complexes with many cations in which the cation is bound in a ring structure. The ring results from the formation of a salt-like bond between the cation and the carboxyl groups together with a coordinate bond through the lone pair of electrons of the nitrogen atom. If the ring is five-membered, the chelate thus formed is likely to have high stability, so the most useful chelating titrants are those that favour the formation of such rings. This is the case with edetic acid (ethylenediaminetetraacetic acid, EDTA); the commonly used disodium salt is known as disodium edetate. With most metals carrying more than unit positive charge, edetic acid forms highly water-soluble 1:1 complexes of such a structure that at least 3 five-membered chelate rings are formed, thus conferring high stability on the complex. In some cases, coordinate bonds other than those resulting from donation of the nitrogen lone pair of electrons may be formed with the carbonyl oxygens of the remaining carboxylic acid groups. Thus the complexes formed by calcium and by trivalent aluminium may be represented:
and
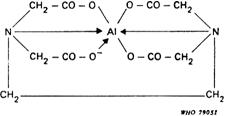
The stability of such complexes is markedly dependent on pH. Most divalent metals form complexes that are stable in alkaline solution but alkaline earth chelates decompose below about pH 8, whereas many divalent metal complexes (zinc and lead, for example) are also stable in quite acid solution. Trivalent metal complexes, by virtue of the additional stability conferred by an increased number of chelate rings, are often stable even in strongly acid solutions. In alkaline solutions, however, some of these metals may be precipitated as hydroxides in the presence of edetic acid, not because of instability of the complex but because of the more powerful effect of the very low solubility product of the metal hydroxide.
Stability constants of the edetic acid chelates of some metals, as recorded by Schwarzenbach for 0.1 mol/l solutions at 20°C are as follows:
|
Na |
1.7 |
|
Li |
2.8 |
|
Mg |
8.7 |
|
Ca |
10.6 |
|
Fe2+ |
14.3 |
|
Al |
15.5a |
|
Zn |
16.1 |
|
Pb |
17.6 |
|
Hg2+ |
20.4 |
|
Fe3+ |
25.1 |
a The aluminium chelate is slow to form so that this metal is usually determined by back-titration.
In order to determine the equivalence point in titration of metal ions with edetic acid, it is necessary to use a suitable indicator that will react to the presence of free metal ions in solution. The indicator originally used by Schwarzenbach for titration of calcium ions was murexide (ammonium purpurate) but this is now rarely used. Perhaps the most widely used of indicators has been Mordant Black 11 (also known under several trade names). This has a blue colour in ammoniacal solution but yields red complexes with many metal ions in such solutions; the metal complexes so formed are generally weaker than the corresponding edetic acid complexes, so that titration with edetates will readily remove the metal from the indicator complex and a colour change to full blue signifies total titration of the metal present in solution. Mordant Black 11 is frequently used as a mixture with methyl orange, which serves to provide a screened (more readily detectable) end-point.
Many other substances have been proposed and used as indicators for complexometric titrations, but the present discussion must be limited to a consideration of those that are of potential value in pharmaceutical applications. Calcon and calconcarboxylic acid give a very sharp colour change from wine-red to full blue when a calcium solution is titrated with sodium edetate in the pH range 12-14. If magnesium is present it is precipitated as hydroxide at this pH and, providing the alkali is added before the indicator, does not interfere. Neither of them, however, is very stable in alkaline solution and they should preferably be added at a late stage in the titration.
Another widely used indicator is xylenol orange; this is a conventional acid-base indicator into which iminodiacetic acid groups have been introduced, thus permitting the substance to act as a metal-complexing indicator. The indicator gives a clear colour change from pink-violet to yellow at the end-point in titrations of aluminium, bismuth, lead, mercury, and zinc, and may be used from pH 2-6 according to the metal being titrated.
Recommended procedures
Aluminium
Dissolve the quantity of substance, accurately weighed, as specified in the monograph, in 2 mL of hydrochloric acid (1 mol/l) VS and 50 mL of water, unless other conditions of solution are given in the monograph. Add 50 mL of disodium edetate (0.05 mol/l) VS and neutralize to methyl red/ethanol TS with sodium hydroxide (1 mol/l) VS. Heat the solution to boiling and maintain in a boiling water-bath for at least 10 minutes, cool, add about 50 mg of xylenol orange indicator mixture R and 5 g of methenamine R and titrate the excess edetate with lead nitrate (0.05 mol/l) VS until the yellow solution turns pink-violet. Each mL of disodium edetate (0.05 mol/l) VS is equivalent to 1.349 mg of Al.
Bismuth
Dissolve the quantity of substance, accurately weighed, as specified in the monograph, in the minimum quantity of nitric acid (~130 g/l) TS, add 50 mL of water and adjust the pH to between 1 and 2 by dropwise addition of either nitric acid (~130 g/l) TS or ammonia (~100 g/l) TS. Add about 50 mg of xylenol orange indicator mixture R and slowly titrate with disodium edetate (0.05 mol/l) VS until the solution turns from pink-violet to a full yellow. Each mL of disodium edetate (0.05 mol/l) VS is equivalent to 10.45 mg of Bi.
Calcium
Dissolve the quantity of substance, accurately weighed, as specified in the monograph, in a few millilitres of water, acidified with a minimum quantity of hydrochloric acid (~70 g/l) TS if necessary, and then dilute to about 100 mL with water. Titrate with disodium edetate (0.05 mol/l) VS to within about 2 mL of the expected equivalence point, add 4 mL of sodium hydroxide (~300 g/l) TS and 0.1 g of calcon indicator mixture R or of calcon carboxylic acid indicator mixture R and continue the titration until the solution turns from pink to a full blue. Each mL of disodium edetate (0.05 mol/l) VS is equivalent to 2.004 mg of Ca.
Lead
Dissolve the quantity of substance, accurately weighed, as specified in the monograph, in 5-10 mL of water, acidified with a minimum quantity of acetic acid (~300 g/l) TS if necessary, and then dilute to about 50 mL with water. Add about 50 mg of xylenol orange indicator mixture R and sufficient methenamine R (about 5 g) to turn the solution red and titrate with disodium edetate (0.05 mol/l) VS until the solution turns from deep violet to full yellow. Each mL of disodium edetate (0.05 mol/l) VS is equivalent to 10.35 mg of Pb.
Magnesium
Dissolve the quantity of substance, accurately weighed, as specified in the monograph, in 5-10 mL of water, acidified with a minimum quantity of hydrochloric acid (~70 g/l) TS if necessary, and then dilute to about 50 mL with water. Add 10 mL of ammonium chloride buffer, pH 10.0, TS and 100 mg of Mordant Black 11 indicator mixture R and titrate with disodium edetate (0.05 mol/l) VS until the solution turns from violet to blue. Each mL of disodium edetate (0.05 mol/l) VS is equivalent to 1.215 mg of Mg.
Zinc
Dissolve the quantity of substance, accurately weighed, as specified in the monograph, in 5-10 mL of water, acidified with a minimum quantity of acetic acid (~300 g/l) TS if necessary, and then dilute to about 50 mL with water. Add about 50 mg of xylenol orange indicator mixture R and sufficient methenamine R (about 5 g) to turn the solution pink-violet and titrate with disodium edetate (0.05 mol/l) VS until the solution turns from pink-violet to full yellow. Each mL of disodium edetate (0.05 mol/l) VS is equivalent to 3.268 mg of Zn.