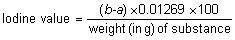Methods of Analysis: 4. Methods for materials of plant origin: 4.2 Determination of iodine value
The iodine value of a substance is the weight of halogens expressed as iodine absorbed by 100 parts by weight of the substance. The quantity of substance used in the determination should be such that at least 70% of the iodine added, as provided in the recommended procedure, is not absorbed. Unless otherwise specified in the monograph, the quantity of the substance indicated in the following table should be used for the determination, depending on the expected iodine value:
|
Iodine value |
Quantity of substance |
|
less than 20 |
1.0 |
|
20 - 60 |
0.5 - 0.25 |
|
60 - 100 |
0.25 - 0.15 |
|
more than 100 |
0.15 - 0.10 |
Recommended procedure
Place a quantity of the test substance, accurately weighed, as specified in the monograph, in a dry 300-mL to 500-mL stoppered flask, add 15 mL of carbon tetrachloride R and dissolve. Add 25 mL of iodine bromide TS, insert the stopper, previously moistened with potassium iodide (80 g/l) TS, shake the flask gently, and keep in the dark for 30 minutes, unless otherwise specified in the monograph. Add 20 mL of potassium iodide (80 g/l) TS and 150 mL of water, and, whilst shaking the contents of the flask, titrate with sodium thiosulfate (0.1 mol/l) VS, adding starch TS as indicator towards the end of the titration. Note the number of mL required (a). At the same time carry out the operation in exactly the same manner, but without the substance being tested, and note the number of mL of sodium thiosulfate (0.1 mol/l) VS required (b). Calculate the iodine value from the following formula: