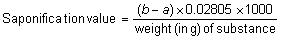Methods of Analysis: 4. Methods for materials of plant origin: 4.4 Determination of saponification value
The saponification value is the number of mg of potassium hydroxide required to neutralize the fatty acids resulting from the complete hydrolysis of 1 g of the substance.
In the procedure described, a 50-mL burette should preferably be used for titration, as in the blank titration the volume of hydrochloric acid (0.5 mol/l) VS used is exactly 35.5 mL when the concentration of ethanolic potassium hydroxide is exactly 40 g/l.
Recommended procedure
Place about 2 g of the test substance, accurately weighed, or the quantity specified in the monograph, in a flask with a capacity of about 200 mL, add 25 mL of potassium hydroxide/ethanol, TS1 attach a reflux condenser, and heat in a boiling water-bath for 30 minutes, or the time specified in the monograph, frequently rotating the contents of the flask; immediately add 1 mL of phenolphthalein/ethanol TS and titrate the excess of alkali with hydrochloric acid (0.5 mol/l) VS. Note the number of mL of hydrochloric acid (0.5 mol/l) VS required to titrate the sample (a). Repeat the operation without the substance being tested and note the number of mL of hydrochloric acid (0.5 mol/l) VS required for neutralization (b). Calculate the saponification value from the following formula: