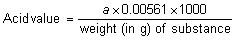Methods of Analysis: 4. Methods for materials of plant origin: 4.6 Determination of acid value
The acid value is the number of mg of potassium hydroxide required to neutralize the free acid in 1 g of the substance.
Recommended procedure
Accurately weigh about 10 g of the substance, or the quantity specified in the monograph, into a 250-mL flask, and add 50 mL of a mixture of equal volumes of ethanol (~750 g/l) TS and ether R, which has been neutralized with potassium hydroxide (0.1 mol/l) VS after the addition of 1 mL of phenolphthalein/ethanol TS. Heat, if necessary, until the substance has completely dissolved, cool; titrate with potassium hydroxide (0.1 mol/l) VS, constantly shaking the contents of the flask until a pink colour, which persists for 15 seconds, is obtained. Note the number of mL required (a). Calculate the acid value from the following formula: