Methods of Analysis: 5. Pharmaceutical technical procedures: 5.4 Disintegration test for suppositories and rectal capsules
The disintegration test determines whether suppositories disintegrate or soften within a prescribed time when placed in an immersion fluid using the experimental conditions described below.
Disintegration is considered to be achieved when:
- dissolution is complete;
- the components of the suppositories have separated, e.g. melted fatty substances have collected on the surface of the liquid, insoluble powders have fallen to the bottom and soluble components have dissolved or are distributed in one or more of the ways described in Methods 1 and 2;
- there is softening of the test sample, usually accompanied by an appreciable change of shape without complete separation of the components. The softening process is such that a solid core no longer exists when pressure is applied with a glass rod; rupture of the gelatin shell or rectal capsule occurs resulting in release of the contents.
Method 1 (for water-soluble, hydrodispersible and fat-based suppositories and rectal capsules)
This test measures the time elapsed for a suppository or rectal capsule placed in water to disintegrate.
Apparatus
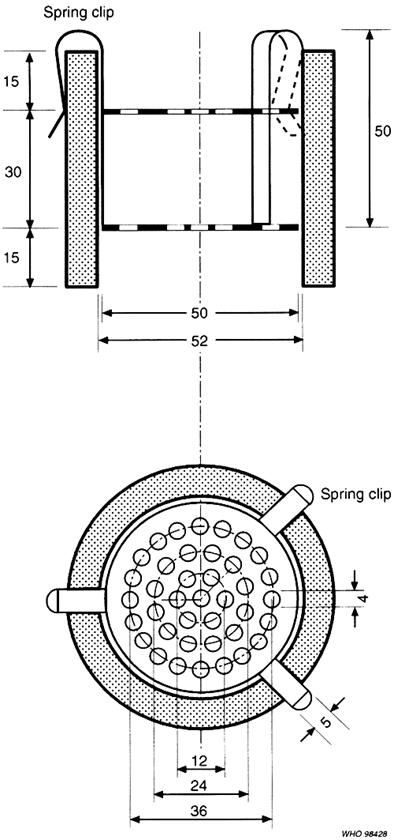
The apparatus (Figure 1) consists of a 60 mm long cylinder of glass or transparent plastic and a metal device consisting of two perforated stainless steel discs held about 30 mm apart. These discs each have 39 holes, 4 mm in diameter, which are evenly spaced in a concentric pattern. The diameter of the discs is marginally inferior to that of the interior of the cylinder. Once inserted into the cylinder, the metal device is attached to the rim of the cylinder by means of three spring clips. The test is carried out using three such apparatuses each containing a single test sample. Each apparatus is placed in a beaker with a minimum capacity of 4 litres filled with water unless otherwise prescribed. The beaker is fitted with a slow stirrer and a support that holds the apparatus vertically 90 mm below the surface of the water so that it can be inverted without emerging from the water.
|
Figure 1. |
Apparatus for water-soluble, hydrodispersible and fat-based suppositories |
|
A. Horizontal view. B. Vertical view. Dimensions in millimetres. |
Procedure
Unless otherwise described in the individual monograph use water maintained at a temperature of 36–37 °C as the immersion fluid. The test requires three suppositories or rectal capsules and the procedure is applied to each of them.
Place the sample on the lower disc of the metal device and then insert it into the cylinder. Place the apparatus into the beaker and invert it every 10 minutes without removing it from the liquid. Repeat the operation with the remaining two suppositories or rectal capsules. Record the time required for the disintegration of the suppositories or rectal capsules.
Unless otherwise stated in the individual monograph for each of the three suppositories or rectal capsules examine the state of the sample after 30 minutes for fat-based suppositories and rectal capsules and after 60 minutes for water-soluble suppositories.
Method 2 (alternative for fat-based suppositories)
This test measures the time elapsed for a suppository placed in water to soften to the extent that it no longer offers resistance when a defined weight is applied. The softening time is determined according to the text Softening time determination of lipophilic suppositories, published in the Supplementary information section.
Apply the procedure to three suppositories and examine the state of each sample after 30 minutes unless otherwise stated in the individual monograph.