Reagents, test solutions and volumetric solutions: C - Cadmium R.... Cytosine R
Cadmium R
Cd
A commercially available reagent of suitable grade.
Cadmium standard (1000 μg Cd/mL) TS
Procedure. Dissolve 0.100 g of cadmium R in sufficient amount of equal volumes of hydrochloric acid (~330 g/L) TS and water R and dilute to 100 mL with a 1% V/V solution of hydrochloric acid (~330 g/L) TS.
Note. For the preparation of this test solution commercially available cadmium standard solution 1000 μg Cd/mL can also be used.
Cadmium acetate R
(CH3CO2)2Cd,2H2O. Contains not less than 98.0% of (CH3CO2)2Cd,2H2O.
Description. Colourless crystals.
Solubility. Soluble in water.
Assay. Dissolve 1 g, accurately weighed, in 50 mL of water, add 25 mL of ammonia (~260 g/L) TS and titrate with disodium edetate (0.1 mol/L) VS, using methylthymol blue mixture R as indicator, until the blue solution becomes colourless or grey. Each mL of disodium edetate (0.1 mol/L) VS is equivalent to 26.65 mg of (CH3CO2)2Cd,2H2O.
Caesium chloride R
CsCl.
A commercially available reagent of suitable grade.
Calcium acetate (0.25 mol/L) VS
Calcium acetate R, dissolved in water to contain 44.04 g of Ca(C2H3O2)2,H2O in 1000 mL.
Calcium acetate R
Ca(C2H3O2)2,H2O (SRIP, 1963, p. 56).
Calcium carbonate R1
CaCO3 (SRIP, 1963, p. 56).
Calcium carbonate R2
CaCO3. Calcium carbonate R1 of suitable quality to serve as a primary standard for the standardization of disodium edetate.
Calcium chloride (3.7g/L) TS
A solution of anhydrous calcum chloride R containing about 9 g of CaCl2 per litre.
Calcium chloride (55 g/L) TS
A solution of hydrated calcium chloride R containing about 55 g/L of CaCl2 (approximately 0.5 mol/L).
Calcium chloride, anhydrous, R
[calcium chloride R] CaCl2 (SRIP, 1963, p. 58).
Calcium chloride, hydrated, R
CaCl2,6H2O (SRIP, 1963, p. 58).
Calcium fluoride R
CaF2.
Description. A white powder.
Solubility. Practically insoluble in water; slightly soluble in dilute acids.
Calcium gluconate R
C12H22CaO14,H2O.
A commercially available reagent of suitable grade.
Calcium hydroxide R
Ca(OH)2 (SRIP, 1963, p. 59).
Calcium hydroxide TS
Procedure. Prepare a saturated solution of calcium hydroxide R.
Note: Calcium hydroxide TS must be freshly prepared.
Calcium standard (10 μg/mL Ca) TS
Procedure. Dissolve 2.50 g of dried calcium carbonate R2 in 15 mL of acetic acid (~300 g/L) TS and dilute with water to 1000 mL (solution A). Dilute 10.0 mL of this solution with water to produce 1000 mL.
Calcium standard (100 μg/mL Ca), ethanolic, TS
Procedure. Dilute 100.0 mL of solution A, described under calcium standard (10 μg/mL Ca) TS, with sufficient ethanol (~750 g/L) TS to produce 1000 mL.
Calcium sulfate R
CaSO4,2H2O (SRIP, 1963, p. 62).
Calcium sulfate TS
Procedure. Shake 5 g of calcium sulfate hemihydrate R for 1 hour with 100 mL of water and filter.
Calcium sulfate, hemihydrate R
Plaster of Paris, CaSO4, 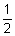 H2O.
H2O.
Description. A white powder which, when mixed with half its weight of water, rapidly solidifies to a hard and porous mass.
Calcon carboxylic acid indicator mixture R
Procedure. Mix 0.1 g of calcon carboxylic acid R with 10 g of anhydrous sodium sulfate R.
Calcon carboxylic acid R
2-Hydroxy-1-(2-hydroxy-4-sulfo-1-naphthyl-azo)-3-naphthoic acid; C21H14N2O7S,3H2O.
Description. A dark-brown powder with a violet tint.
Solubility. Practically insoluble in water; slightly soluble in methanol R and in ethanol (~750 g/L) TS; freely soluble in solutions of alkali hydroxides.
Calcon indicator mixture R
Procedure. Mix 0.1 g of calcon R with 10 g of anhydrous sodium sulfate R.
Calcon R
Monosodium salt of 2-hydroxy-1-[(2-hydroxy-1-naphthyl)-azo]naphthalene-4-sulfonic acid; C.I. Mordant Black 17, C.I. No. 15705, Eriochrome Blue Black R, Solochrome Dark Blue; C20H13N2NaO5S.
Carbazole R
Dibenzopyrrole; C12H9N.
A commercially available reagent of suitable grade.
Melting point. about 245 °C.
Carbomer R
Carbomer suitable for thin-layer chromatography. A high relative molecular mass cross-linked polymer of acrylic acid; it contains a large proportion (56-68%) of carboxylic acid (-COOH) groups after drying at 80 °C for 1 hour.
pH value. The pH of a 10 g/L suspension is about 3.
Viscosity. Whilst stirring continuously prepare a suspension containing 2.5 g in 500 mL of water. Maintain at 25 ± 0.2 °C for 30 minutes then add 0.2 mL of phenolphthalein/ethanol TS, 1 mL of bromothymol blue/ethanol TS and, whilst stirring, neutralize using a mixture of equal volumes of sodium hydroxide (~400 g/L) TS and water until a uniform blue colour is obtained (check the pH which must be 7.3–7.8). The dynamic viscosity of the neutralized preparation is 30–40 Pa s (300–400 poise).
Carbon dioxide detector tube
A cylindrical, sealed glass tube containing adsorbent filters and suitable supports for hydrazine and crystal violet indicators. The minimum value indicated is 100 μl/L or less, with a relative standard deviation of at most ±15%. Tubes can be verified with a calibration gas containing the appropriate impurity if a negative result is obtained.
Carbon dioxide R
CO2.
Description. A colourless gas; odourless.
Solubility. Soluble in about 1.3 parts by volume of water.
Carbon disulfide IR
Carbon disulfide R that complies with the following test: The infrared absorption spectrum of a 1.0 mm layer, as described in method 4 under 1.7 Spectrophotometry in the infrared region and examined over the range 4000–670 cm-1 shows an absorbance of less than 0.1 in the regions 4000–3030 cm-1, 2635–2440 cm-1, 2000–1755 cm-1, and 1265–935 cm-1, and an absorbance of less than 0.17 in the region 800–715 cm-1.
Carbon disulfide R
CS2 (SRIP, 1963, p. 62).
Carbon monoxide detector tube
A cylindrical, sealed glass tube containing adsorbent filters and suitable supports for di-iodine pentoxide, selenium dioxide and fuming sulfuric acid indicators. The minimum value indicated is 5 μl/L or less with a relative standard deviation of at most ±15%. Tubes can be verified with a calibration gas containing the appropriate impurity if a negative result is obtained.
Carbon monoxide R
CO.
A commercially available gas of suitable grade.
Carbon tetrachloride R
CCl4 (SRIP, 1963, p. 63).
Carboxymethylcellulose R
A suitable grade for column chromatography.
Cefadroxil R
Cefadroxil of a suitable quality should be used.
Cellulose R1
Microcrystalline cellulose suitable for thin-layer chromatography.
Description. A fine, white homogeneous powder.
Particle size. Less than 30 μm.
Note: A suspension of about 25 g of cellulose R1 in 90 mL of water is used in the preparation of the coating for thin-layer chromatographic plates.
Cellulose R2
Cellulose suitable for thin-layer chromatography.
Description. A fine, white homogeneous powder.
Particle size. Less than 30 μm.
Note: A suspension of about 15 of cellulose R2 in 100 mL of water is used in the preparation of the coating for thin-layer chromatographic plates.
Cellulose R3
Cellulose suitable for thin-layer chromatography.
Description. A fine, white homogeneous powder.
Composition. Cellulose (particle size less than 30 μm) containing a fluorescent indicator having an optimal intensity at 254 nm.
Note: A suspension of about 25 g of cellulose R3 in 100 mL of water is used in the preparation of the coating for thin-layer chromatographic plates.
Cefadroxil R
(6R,7R)-7-{[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. C16H17N3O5S.
A commercially available reagent of suitable grade.
Cephaëline hydrochloride R
C28H38N2O4,2HCl,7H2O.
Description. A white, crystalline powder.
Specific optical rotation. Use a 20 mg/mL solution; 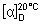 = +25°.
= +25°.
Cephalin TS
Procedure. Place a quantity between 0.5 and 1.0 g of acetone-dried ox brain R into a centrifuge tube, add 20 mL of acetone R and allow to stand for 2 hours. Centrifuge for 2 minutes and decant the supernatant liquid. Dry the residue under reduced pressure, add to it 20 mL of chloroform R and allow to stand for 2 hours, shaking frequently. Separate the solid material by filtration or centrifugation and evaporate the chloroform under reduced pressure. Suspend the residue in 5–10 mL of sodium chloride (9 g/L) TS. Solvents used to prepare cephalin TS should contain a suitable antioxidant, for example, a solution of 0.02 g/L of butylated hydroxyanisole R.
Storage. Store in a freezer or keep in a freeze-dried state.
Note : The reagent must be used within 3 months.
Ceric ammonium nitrate (0.01 mol/L) VS
Procedure. Dissolve 5.482 g of ceric ammonium nitrate R in sufficient nitric acid (1 mol/L) VS to produce 1000 mL and filter.
Method of standardization. Ascertain the exact concentration of the 0.01 mol/L solution in the following manner: measure accurately 2.0 mL of freshly standardized ferrous ammonium sulfate (0.1 mol/L) VS into a flask and dilute with water to about 100 mL. Add 1 drop of nitrophenanthroline TS and titrate with the ceric ammonium nitrate solution to a colourless end-point. From the volume of ferrous ammonium sulfate (0.1 mol/L) VS taken and the volume of ceric ammonium nitrate solution consumed, calculate the molarity.
Ceric ammonium nitrate R
Ce(NO3)4,2NH4NO3.
Description. Small orange-red monoclinic crystals.
Solubility. Very soluble in water.
Insoluble matter. To 5 g, accurately weighed, add 10 mL of sulfuric acid (~1760 g/L) TS, stir and cautiously add 90 mL of water to dissolve. Heat to boiling and digest in a covered beaker on a water-bath for 1 hour. Filter through a tared filtering crucible, wash thoroughly and dry at 105 °C. The weight of the residue does not exceed 2.5 mg.
Assay. Dissolve 2.5 g, accurately weighed and previously dried at 85 °C for 24 hours, in 10 mL of sulfuric acid (~190 g/L) TS and add 40 mL of water. Add a few drops of o-phenanthroline TS and titrate with ferrous sulfate (0.1 mol/L) VS. Each mL of ferrous sulfate (0.1 mol/L) VS is equivalent to 54.8 mg of Ce(NO3)4,2NH4NO3.
Ceric ammonium sulfate (0.1 mol/L) VS
Procedure. Dissolve 65.0 g of ceric ammonium sulfate R in a mixture of 500 mL of water and 30 mL of sulfuric acid (~1760 g/L) TS. Allow to cool and dilute to 1000 mL with water.
Method of standardization. Ascertain the exact concentration of the 0.1 mol/L solution in the following manner: accurately weigh about 0.2 g of arsenic trioxide R1 and dissolve by gently heating in 15 mL of sodium hydroxide (0.2 mol/L) VS. Add to the clear solution 50 mL of sulfuric acid (~100 g/L) TS, 0.15 mL of a 2.5 mg/mL solution of osmium tetroxide R in sulfuric acid (~100 g/L) TS and 0.1 mL of o-phenanthroline TS. Titrate the solution with the ceric ammonium sulfate solution until the red colour disappears. Titrate slowly as the end-point is approached.
Ceric ammonium sulfate R
Ammonium cerium (IV) sulfate dihydrate, Ce(SO4)2,2(NH4)2SO4,2H2O. Contains not less than 95.0% of Ce(SO4)2,2(NH4)2SO4,2H2O.
Description. Yellow crystals or an orange-yellow, crystalline powder.
Solubility. Slowly soluble in water; insoluble in ethanol (~750 g/L) TS.
Assay. Dissolve about 1 g, accurately weighed, in 50 mL of sulfuric acid (~100 g/L) TS, add 0.1 mL of a 10 mg/mL solution of osmium tetroxide R and titrate with sodium arsenite (0.05 mol/L) VS using o-phenanthroline TS as indicator. Each mL of sodium arsenite (0.05 mol/L) VS is equivalent to 63.26 mg of Ce(SO4)2,2(NH4)2SO4,2H2O.
Ceric ammonium sulfate/nitric acid TS
Procedure. Dissolve 5 g of ceric ammonium sulfate R in sufficient nitric acid (~130 g/L) TS to produce 100 mL.
Ceric amonium nitrate TS
Procedure. Dissolve 6.25 g of ceric ammonium nitrate R in 10 mL of nitric acid (15 g/L) TS.
Shelf-life. Use within 3 days of preparation.
Ceric sulfate (0.1 mol/L) VS
Procedure. Dissolve ceric sulfate R, equivalent to 33.23 g of Ce(SO4), in a mixture of 28 mL of sulfuric acid (~1760 g/L) TS and 500 mL of water, dilute to 1000 mL and mix. Allow the solution to stand for 48 hours and filter through a sintered glass filter.
Method of standardization. Ascertain the exact concentration of the 0.1 mol/L solution in the following manner: place about 25 mL, accurately measured, in a glass-stoppered flask, dilute with 80 mL of water, add 10 mL of phosphoric acid (~105 g/L) TS and 2.5 g of potassium iodide R and allow the solution to stand for 15 minutes. Add 1 g of sodium carbonate R and titrate with sodium thiosulfate (0.1 mol/L) VS, using starch TS as indicator.
Ceric sulfate (35 g/L) TS
A solution of ceric sulfate R containing about 33 g/L of Ce(SO4)2.
Ceric sulfate R
Usually Ce(SO4)2,4H2O (SRIP, 1963, p. 63).
Charcoal R
(SRIP, 1963, p. 64).
Chloralose R
C8H11Cl3O6.
Description. A colourless, crystalline powder.
Melting temperature. About 187 °C.
Specific optical rotation. Use a 50 mg/mL solution in ethanol (~750 g/L) TS; 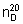 = +19°.
= +19°.
Chloraniline R
4-Chloroaniline, C6H6ClN.
Description. White or faintly coloured crystals.
Melting temperature. About 70 °C.
Chlorbutol R
A commercially available reagent of suitable grade
Chloride standard (5 μg/L) TS
Procedure. Weigh accurately 82.4 mg of sodium chloride R and dissolve in sufficient water to produce 100 mL. Dilute 1.0 mL of this solution with water to 100 mL.
Chlorine R
Cl2 (SRIP, 1963, p. 65).
Chlorine TS
A saturated solution of chlorine R in water.
Note: Chlorine TS must be freshly prepared.
7-Chloro-1-cyclopropyl-1,4-dihydro-4-oxo-6-(piperazin-1-yl)quinoline-3-carboxylic acid RS
(ciprofloxacin by-compound A). International Chemical Reference Substance.
2-Chloro-2-deoxy-D-glucose R
C6H11ClO5.
Description. A white crystalline, hygroscopic powder, soluble in water and in dimethyl sulphoxide and insoluble in alcohol.
A commercially available reagent of suitable grade.
1-Chloro-2,4 dinitrobenzene R
C6H3ClN2O4.
A commercially available reagent of suitable grade.
Melting point. About 144 °C.
1-Chloro-2,4 dinitrobenzene/ethanol TS
Procedure. Weigh 5 g of 1-Chloro-2,4 dinitrobenzene R and dissolve in sufficient ethanol (~750 g/L) TS to produce 100 mL.
2-Chloro-4-nitroaniline R
C6H5ClN2O2.
Description. A yellow to brown, crystalline powder.
Solubility. Slightly soluble in water; soluble in ethanol (~750 g/L) TS.
Melting range. 106–108 °C.
Sulfated ash. Not more than 0.5 mg/g.
4-Chloroacetanilide R
C8H8ClNO.
Description. Colourless, needle-shaped crystals or a white to pale yellow, crystalline powder.
Solubility. Practically insoluble in water; soluble in ethanol (~750 g/L) TS and ether R.
Melting temperature. About 180 °C.
Chloroform R
CHCl3 (SRIP, 1963, p. 66).
Chloroform, ethanol-free, R
Procedure. Shake 20 mL of chloroform R gently but thoroughly with 20 mL of water for 3 minutes, draw off the chloroform layer and wash twice more with 20 mL quantities of water. Finally filter the chloroform through a dry filter-paper, shake it well with 5 g of powdered anhydrous sodium sulfate R for 5 minutes, allow the mixture to stand for 2 hours and decant or filter the clear chloroform.
Chromazurol S R
C23H13Cl2NaO9S.
Description. A brownish-black powder.
A commercially available reagent of suitable grade.
Solubility. Soluble in water; slightly soluble in alcohol.
Chromic acid TS
Procedure. Dissolve 84 g of chromium trioxide R in 700 mL of water and add slowly while stirring 400 mL of sulfuric acid (~1760 g/L) TS.
Chromium trioxide R
CrO3 (SRIP, 1963, p. 68).
Cinchonidine R
C19H22N2O.
Description. A white, crystalline powder.
Solubility. Soluble in ethanol (~750 g/L) TS.
Melting temperature. About 207 °C.
Specific optical rotation. Use a 50 mg/mL solution in ethanol (~750 g/L) TS; 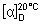 = -105° to 110°.
= -105° to 110°.
Cinchonine R
C19H22N2O (SRIP, 1963, p. 69).
Citrate buffer, pH 4.0, TS
Procedure. Dissolve 10.5 g of citric acid R in about 100 m of water, add 100 m of sodium hydroxide (1 mol/L) VS and dilute to 500 mL with water. Dilute 100 mL of hydrochloric acid (0.1 mol/L) VS with the solution prepared above to produce 250 mL.
Citrate buffer, pH 5.4, TS
Procedure. Dissolve 2.101 g of citric acid R in water, add 20 mL of sodium hydroxide (1 mol/L) VS and dilute with sufficient water to produce 100 mL. Mix 76.5 mL of this solution with 23.5 mL of sodium hydroxide (0.1 mol/L) VS.
Citrate buffer, pH 5.0, TS
Procedure. Dissolve 20.17 g of citric acid R in 800 mL of water R, adjust to pH 5.0 with sodium hydroxide (~400 g/L) TS and dilute to 1000 mL with water R.
Citrate buffer, pH 5.4, TS
Procedure. Dissolve 2.101 g of citric acid R in water, add 20 mL of sodium hydroxide (1 mol/L) VS and dilute with sufficient water to produce 100 mL. Mix 76.5 mL of this solution with 23.5 mL of sodium hydroxide (0.1 mol/L) VS.
Citric acid (180 g/L) FeTS
A solution of citric acid FeR containing about 183 g/L of C6H8O7.
Citric acid (20 g/L) TS
A solution of citric acid R containing about 20 g of C6H8O7 per litre.
Citric acid FeR
Citric acid R that complies with the following test: Dissolve 0.5 g of citric acid R in 40 mL of water, add 2 drops of mercaptoacetic acid R, mix, make alkaline with ammonia (~100 g/L) FeTS and dilute to 50 mL with water; no pink colour is produced.
Citric acid PbR
Citric acid R free of lead.
Citric acid R
C6H8O7,H2O (SRIP, 1963, p. 69).
Citric acid, copper-free, R
Citric acid R, that complies with the following additional test: Dissolve 0.50 g in 20 mL of water, make alkaline with ammonia (~100 g/L) TS, dilute to 50 mL with water and add 1 mL of sodium diethyldithiocarbamate (0.8 g/L) TS; no yellow colour is produced.
Cobalt colour TS
A solution containing 60.0 g/l of CoCl2,6H2O.
Procedure. Prepare a solution containing 6.000 g of CoCl2,6H2O in 100 mL by diluting the strong cobalt colour TS with sulfuric acid (~10 g/L) TS, as necessary.
Cobalt colour, strong, TS
Procedure. Dissolve 8.0 g of cobaltous chloride R in 120 mL of sulfuric acid (~10 g/L) TS, filter the solution if necessary and determine the concentration of CoCl2,6H2O.
Assay. Dilute 5.0 mL with sufficient water to produce 100 mL. Transfer 10.0 mL of this solution to a glass-stoppered flask, add 10 mL of water, 0.5 mL of hydrogen peroxide (~60 g/L) TS and 10 mL of sodium hydroxide (~80 g/L) TS. Add a few boiling chips to the flask and boil the contents of the flask until the excess of hydrogen peroxide is completely decomposed (approximately 10 minutes). Cool the flask, add 20 mL of water, 1 g of potassium iodide R and 25 mL of hydrochloric acid (2 mol/L) VS. Close the flask with the stopper and allow to stand until the precipitate dissolves. Titrate the liberated iodine with sodium thiosulfate (0.01 mol/L) VS using starch TS as indicator. Each mL of sodium thiosulfate (0.01 mol/L) VS is equivalent to 2.380 mg of CoCl2,6H2O.
Cobalt(II) chloride (30 g/L) TS
A solution of cobalt(II) chloride R containing about 30 g of CoCl2 per litre.
Cobalt(II) chloride (5 g/L) TS
A solution of cobalt(II) chloride R containing about 5 g of CoCl2 per litre.
Cobalt(II) chloride R
Cobaltous chloride; CoCl2,6H2O (SRIP, 1963, p. 70).
Cobalt(II) nitrate (10 g/L) TS
Procedure. Dissolve about 1.6 g of cobalt(II) nitrate R in sufficient water to produce 100 mL.
Cobalt(II) nitrate (100 g/L) TS
A solution of cobalt(II) nitrate R containing about 100 g of Co(NO3)2 per litre.
Cobalt(II) nitrate R
Co(NO3)2,6H2O.
Description. Small red crystals.
Solubility. Very soluble in water.
Cobaltous chloride R
CoCl2,6H2O (SRIP, 1963, p. 70).
Cobaltous chloride TS
Procedure. Dissolve 6.5 g of cobaltous chloride R in a sufficient quantity of a mixture of 2.5 mL of hydrochloric acid (~250 g/L) TS and 97.5 mL of water to produce 100 mL.
Codeine R
C18H21NO3,H2O.
Description. Colourless crystals or a white, crystalline powder; odourless.
Solubility. Slightly soluble in water; freely soluble in ethanol (~750 g/L) TS and ether R.
Melting temperature. About 156 °C.
Specific optical rotation. Use a 20 mg/mL solution in ethanol (~750 g/L) TS; 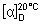 = -142° to -146°.
= -142° to -146°.
Colbatous thiocyanate TS
Procedure. Dissolve 6.8 g of cobaltous chloride R and 4.3 g of ammonium thiocyanate R in sufficient water to produce 100 mL.
Congo red paper R
(SRIP, 1963, p. 72).
Copper colour TS
A solution containing 60.0 g/L of CuSO4,5H2O.
Procedure. Prepare a solution containing 6.000 g of CuSO4,5H2O in 100 mL by diluting the strong copper colour TS with sulfuric acid (~10 g/L) TS as necessary.
Copper colour, strong, TS
Procedure. Dissolve 8.0 g of copper(II) sulfate R in 120 mL of sulfuric acid (~10 g/L) TS, filter the solution if necessary and determine the concentration of CuSO4,5H2O.
Assay. Dilute 5.0 mL with sufficient water to produce 100 mL. Transfer 10.0 mL of this solution to a glass-stoppered flask, add 20 mL of water, 1 g of potassium iodide R and 5 mL of glacial acetic acid R. After 10 minutes titrate the liberated iodine with sodium thiosulfate (0.01 mol/L) VS using starch TS as indicator. Each mL of sodium thiosulfate (0.01 mol/L) VS is equivalent to 2.497 mg of CuSO4,5H2O.
Copper edetate TS
Procedure. To 2 mL of a 20 mg/mL solution of copper(II) acetate R add 2 mL of disodium edetate (0.1 mol/lLVS and dilute to 50 mL with water.
Copper standard (10 μg/mL Cu) T.
Procedure. Dissolve 0.393 g of copper(II) sulfate R in sufficient water to produce 100 mL and dilute 10.0 mL of this solution to produce 1000 mL.
Copper standard (5 µg/mL Cu) TS
Procedure. Dissolve 0.982 g of copper(II) sulfate R in 1000 mL of hydrochloric acid (0.1 mol/L) VS. Transfer 2.0 mL of this solution to a 100 mL volumetric flask, dilute to volume with hydrochloric acid (0.1 mol/L) VS and mix. Each mL of this solution contains 5 µg of copper.
Copper standard TS1
Procedure. Dissolve 1.965 g of copper(II) sulfate R, accurately weighed, in sufficient hydrochloric acid (0.1 mol/L) VS to produce 1000 mL.
Copper standard TS2
Procedure. Transfer 3.0 mL of copper standard TS1 to a 1000 mL flask and dilute with hydrochloric acid (0.1 mol/L) VS to produce 1000 mL. This solution contains 1.5 μg of Cu per mL.
Copper tetramine hydroxide TS
Procedure. Dissolve 34.5 g of copper(II) sulfate R in 100 mL of water. Stir and add, drop by drop, ammonia (~260 g/L) TS until the precipitate formed has completely dissolved. Keep the temperature below 20 °C and add slowly, while stirring, 30 mL of sodium hydroxide (~400 g/L) TS. Filter the precipitate through a sintered glass filter (porosity 16–40 μm) and wash with water until the filtrate is clear. Add 200 mL of ammonia (~260 g/L) TS to the precipitate, stir and filter.
Copper(II) acetate (45 g/L) TS
A solution of copper(II) acetate R containing about 50 g of C4H6CuO4,H2O per litre.
Copper(II) acetate R
C4H6CuO4,H2O. Contains not less than 98.0% of C4H6CuO4,H2O.
Description. Blue-green crystals or powder; odour, resembling that of acetic acid.
Solubility. Soluble in water.
Assay. Dissolve 0.8 g, accurately weighed, in 50 mL of water, add 2 mL of acetic acid (~300 g/L) TS and 3 g of potassium iodide R and titrate the liberated iodine with sodium thiosulfate (0.1 mol/L) VS using starch TS as indicator, until only a faint blue colour remains; add 2 g of potassium thiocyanate R and continue the titration until the blue colour disappears. Each mL of sodium thiosulfate (0.1 mol/L) VS is equivalent to 19.97 mg of C4H6CuO4,H2O.
Copper (I) bromide R
CuBr; 143.45; [7787-70-4]
Description. Pale green powder.
Use a suitable grade.
Copper(II) chloride R
CuCl2,2H2O.
Description. Bluish green, deliquescent crystals.
Solubility. Freely soluble in water; soluble in ethanol (~750 g/L) TS; slightly soluble in ether R.
Copper(II) chloride/ammonia TS
Procedure. Dissolve 22.5 g of copper(II) chloride R in 200 mL of water and add 100 mL of ammonia (~260 g/L) TS.
Copper(II) sulfate (1 g/L) TS
A solution of copper(II) sulfate R containing 1 g of CuSO4 per litre.
Copper(II) Sulfate (10 g/L) TS
A solution of Copper(II) sulfate R containing 10 g of CuSO4 per litre.
Copper(II) sulfate (160 g/L) TS
A solution of copper(II) sulfate R containing about 160 g/L of CuSO4.
Copper(II) sulfate (80 g/L) TS
A solution of copper(II) sulfate R containing about 80 g of CuSO4 per litre (approximately 0.5 mol/L).
Copper(II) sulfate R
CuSO4,5H2O (SRIP, 1963, p. 72).
Copper (II) sulfate R, anhydrous
CuSO4.
Description. Greenish-grey powder, hygroscopic.
Solubility. Freely soluble in water, slightly soluble in methanol and practically insoluble in ethanol (~750 g/L) TS.
Copper (II) sulfate pentahydrate R
CuSO4,5H2O, [7758-99-8]; blue, crystalline powder or transparent, blue crystals, content: 99.0% to 101.0%.
Copper(II) sulfate/ammonia TS
Procedure. Dissolve 50 g of copper(II) sulfate R in 1000 mL of ammonia (~35 g/L) TS.
Copper(II) sulfate/pyridine TS
Procedure. Dissolve 4 g of copper(II) sulfate R in 90 mL of water and add 30 mL of pyridine R.
Note: Copper(II) sulfate/pyridine TS must be freshly prepared.
o-Cresol R
2-Methylphenol; C7H8O.
Description. Colourless to pale brownish-yellow crystals or liquid; odour, resembling that of phenol.
Miscibility. Miscible with ethanol (~750 g/L) TS and ether R; miscible with about 50 parts of water.
Mass density. ρ20 = about 1.05 kg/L.
Refractive index. 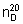 = 1.540–1.550.
= 1.540–1.550.
Boiling temperature. About 190 °C.
Freezing temperature. Not below 30.5 °C.
Residue on evaporation. Evaporate on a water-bath and dry to constant weight at 105 °C; it leaves a residue of not more than 1.0 mg/mL.
Storage. Store in a tightly closed container, protected from light and oxygen.
Note: On exposure to light and air o-cresol R darkens in colour.
Cresol red R
C21H18O5S.
Description. A red-brown powder.
Solubility. Slightly soluble in water; soluble in ethanol (~750 g/L) TS and in dilute solutions of alkali hydroxides.
Cresol red/ethanol TS
Procedure. Warm 0.05 g of cresol red R with 2.65 mL of sodium hydroxide (0.05 mol/L) VS and 5 mL of ethanol (~710 g/L) TS; after solution has been effected add sufficient ethanol (~150 g/L) TS to produce 250 mL.
Crystal violet R
C25H30ClN3 (SRIP, 1963, p. 73).
Crystal violet/acetic acid TS
A solution of crystal violet R dissolved in glacial acetic acid R1 containing about 5 g/L.
Crystal violet/acetic acid TS1
A solution of crystal violet R dissolved in anhydrous acetic acid R containing about 5 g/L.
Test for sensitivity. To 50 mL of anhydrous acetic acid R, add 0.1 mL of the crystal violet solution. On addition of 0.1 mL of perchloric acid (0.1 mol/L) VS, the bluish-purple solution turns bluish-green.
Culture medium Cm1
Procedure. Dissolve 6.0 g of dried peptone R, 4.0 g of pancreatic digest of casein R, 3.0 g of water-soluble yeast extract R, 1.5 g of beef extract R, 1.0 g of glucose hydrate R and 10–20 g of agar R in sufficient water to produce 1000 mL.
Note: The quantity of agar R used should permit the culture medium to be of adequate firmness to support cylinders or to permit holes to be cut without tearing the gel layer.
Culture medium Cm2
Procedure. Dissolve 17.0 g of pancreatic digest of casein R, 3.0 g of papaic digest of soybean meal R, 5.0 g of sodium chloride R, 2.5 g of dipotassium hydrogen phosphate R, 2.5 g of glucose hydrate R and 10–20 g of agar R in about 500 mL of water. Heat the solution, add 10.0 g of polysorbate 80 R and dilute immediately with a sufficient amount of water to produce 1000 mL.
Note: The quantity of agar R used should permit the culture medium to be of adequate firmness to support cylinders or to permit holes to be cut without tearing the gel layer.
Culture medium Cm3
Procedure. Dissolve 9.4 g of dried peptone R, 4.7 g of water-soluble yeast extract R, 2.4 g of beef extract R, 10.0 g of sodium chloride R, 10.0 g of glucose hydrate R and 15–25 g of agar R in sufficient water to produce 1000 mL.
Note: The quantity of agar R used should permit the culture medium to be of adequate firmness to support cylinders or to permit holes to be cut without tearing the gel layer.
Culture medium Cm8
Procedure. Dissolve 10 g of dried peptone R, 10 g of beef extract R, 10 g of glycerol R, 3.0 g of sodium chloride R and 17 g of agar R in sufficient water to produce 1000 mL. Adjust the pH with sodium hydroxide (0.05 mol/L) VS to 6.9–7.1, and sterilize in an autoclave at 121 °C for 18–20 minutes.
Culture medium Cm9
Procedure. Dissolve 10 g of dried peptone R, 10 g of beef extract R, 10 g of glycerol R and 3.0 g of sodium chloride R in sufficient water to produce 1000 mL. Adjust the pH with sodium hydroxide (0.05 mol/L) VS to 6.9–7.1, and sterilize in an autoclave at 121 °C for 18–20 minutes.
Cupri-tartaric TS
Procedure. Dissolve 34.6 g copper (II) sulfate R in sufficient water to produce
100 mL. Separately dissolve 173 g of potassium sodium tartrate R and 50 g sodium hydroxide in 400 mL water R; heat to boiling, allow to cool and dilute to 500 mL with water R. Shortly before use mix together equal volumes of both solutions.
Cyanide/oxalate/thiosulfate TS
Procedure. To 2.0 mL of ammonia (~100 g/L) TS, add in the following order: 1.5 mL of ammonium oxalate (50 g/L) TS, 15 mL of potassium cyanide (50 g/L) TS, 45 mL of sodium acetate (60 g/L) TS, 120 mL of sodium thiosulfate (320 g/L) TS, 75 mL of sodium acetate (60 g/L) TS and 35 mL of hydrochloric acid (1 mol/L) VS.
Note: Cyanide/oxalate/thiosulfate TS must be prepared immediately before use.
Cyanoethylmethyl silicone gum R
A suitable grade to be used in gas-liquid chromatography.
Cyanogen bromide TS
Caution. Very toxic; avoid inhalation of vapours.
Procedure. Add drop by drop, while cooling, potassium cyanide (100 g/L) TS to bromine TS1 until the colour disappears.
Note: Cyanogen bromide TS must be prepared immediately before use.
Cyclohexane R
C6H12 (SRIP, 1963, p. 74).
Cyclohexane R1
Cyclohexane R showing a fluorescence that is not more intense than that of a solution of 2 μg/mL of quinine R in sulfuric acid (0.05 mol/L) VS when measured at 460 nm using a 1 cm layer and an excitation beam at 365 nm.
Cyclohexylenedinitrilotetra-acetic acid R
trans-Cyclohexylene-1,2-dinitrilo-N,N,N’,N’-tetra-acetic acid, C14H22N2O8,H2O.
Description. A white or almost white, crystalline powder.
Melting point. About 204 °C.
3-Cyclohexylpropionic acid R
C9H16O2
Molecular weight.156.2.
Description. Clear liquid.
Relative density![]() . About 0.998.
. About 0.998.
Boiling point.About 130 °C.
L-Cystine R
C6H12N2O4S2 (SRIP, 1963, p. 75).
Cytosine R
4-Aminopyrimidin-2(1H)-one; C4H5N3O.
A commercially available reagent of suitable grade.