Reagents, test solutions and volumetric solutions: H - Helium R.... Hypoxanthine R
Helium R
He. Contains not less than 999.95 mL/L of He.
Heparin RS
World Health Organization International Reference Material. Heparin, porcine, mucosal. 5th International Standard 1998. (Ampoules containing 2031 IU (distributed by the National Institute for Biological Standards and Control (NIBSC), PO Box 1193, Blanche Lane, South Mimms, Potters Bar, Herts EN6 3QH, England.))
Heparinized saline TS
A sterile solution of saline TS containing 50 International Units of heparin in 1 mL.
Heptane R
C7H16 (SRIP, 1963, p. 94).
Hexamethyldisilazane R
C6H19NSi2.
Description.A clear, colourless liquid, having a characteristic odour.
Mass density.ρ20 = about 0.77 kg/L.
Hexane R
n-Hexane, C6H14.
Description.A colourless, mobile, highly inflammable liquid.
Boiling range.Distils completely over a range of 1 °C between 67.5 and 69.5 °C.
Mass density.ρ20 = 0.658-0.659 kg/L
Refractive index.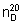 = 1.374–1.375.
= 1.374–1.375.
Hexylamine R
Hexaneamine; C6H15N.
A commercially available reagent of suitable grade.
Description. A colourless liquid.
Boiling point. 127–131 °C.
Refractive index. 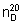 = about 1.418.
= about 1.418.
Mass density. ρ20 = about 0.766 kg/L.
Histamine dihydrochloride R
C5H9N3,2HCl. Contains not less than 98.0% and not more than 101.0% of C5H9N3,2HCl, calculated with reference to the dried substance.
Description.Colourless crystals or a white crystalline powder; odourless.
Solubility.Freely soluble in water and in methanol R; soluble in ethanol (~750 g/L) TS.
Melting range.244–246 °C.
Loss on drying.Not more than 5.0 mg/g.
Assay.Dissolve about 0.15 g, accurately weighed, in 10 mL of water. Add 5 mL of chloroform R and 25 mL of ethanol (~750 g/L) TS. Titrate with carbonate-free sodium hydroxide (0.2 mol/L) VS using 0.5 mL of thymolphthalein/ethanol TS as indicator. Each mL of carbonate-free sodium hydroxide (0.2 mol/L) VS is equivalent to 9.21 mg of C5H9N3,2HCl.
Histamine phosphate R
C5H9N3,2H3PO4. Contains not less than 98.0% and not more than 101.0% of C5H9N3,2H3PO4, calculated with reference to the anhydrous substance.
Description.Colourless, long, prismatic crystals; odourless. Stable in air.
Solubility.Soluble in about 5 parts of water; slightly soluble in ethanol (~750 g/L) TS.
Melting temperature.About 132 °C.
Water.Determined by the Karl Fischer method using about 1.0 g; the water content is 50–60 mg/g.
Assay.Dissolve about 0.15 g, accurately weighed, in 10 mL of water. Add 5 mL of chloroform R and 25 mL of ethanol (~750 g/L) TS. Titrate with carbonate-free sodium hydroxide (0.2 mol/L) VS using 0.5 mL of thymolphthalein/ethanol TS as indicator. Each mL of carbonate-free sodium hydroxide (0.2 mol/L) VS is equivalent to 15.36 mg of C5H9N3,2H3PO4.
Histamine TS
A solution containing 1.0 mg/L of histamine base.
Procedure.Prepare histamine TS by diluting strong histamine TS with a sufficient quantity of saline TS.
Note:Histamine TS must be freshly prepared.
Histamine, strong, TS
A solution containing 1.00 g/L of histamine base.
Procedure.Dissolve 138.1 mg, accurately weighed, of histamine phosphate R or 82.8 mg, accurately weighed, of histamine dihydrochloride R in sufficient water to produce 50.0 mL.
Storage.Strong histamine TS should be stored at a temperature not exceeding 4–10 °C, in dark glass bottles with ground-glass stoppers, protected from light.
Shelf-life.Do not use longer than 30 days.
Holmium oxide R
Ho2O3. Contains not less than 99.9% of Ho2O3, the impurities consisting of Er2O3 and Dy2O3.
Description.A tan-coloured powder.
Solubility.Insoluble in water.
Holmium perchlorate TS
Procedure.Dissolve 40 g of holmium oxide R in sufficient perchloric acid (~140 g/L) TS to produce 1000 mL.
Hydrazine hydrate R
N2H4,H2O. Contains not less than 98.0% of N2H4,H2O.
Description.A clear, colourless liquid.
Miscibility.Miscible with water.
Residue on evaporation.Evaporate to dryness on a water-bath; it leaves a residue of not more than 5.0 mg/g.
Assay.Dilute 1 g to 200 mL with water. Neutralize 20 mL of this solution with hydrochloric acid (~420 g/L) TS and add 10 mL in excess. Add 5 mL of potassium cyanide (100 g/L) TS, titrate with potassium iodate (0.05 mol/L) VS until the brown colour which first forms becomes pale, add starch TS and continue the titration until the blue colour disappears. Each mL of potassium iodate (0.05 mol/L) VS is equivalent to 2.503 mg of N2H4,H2O.
Hydrazine sulfate R
(NH4)2,H2SO4.
Description.Colourless crystals or a white, crystalline powder.
Solubility.Soluble in about 40 parts of water; practically insoluble in ethanol (~750 g/L) TS.
Arsenic. Use a solution of 10 g in 35 mL of boiling water and proceed as described under 2.2.5 Limit test for arsenic; not more than 1 μg/g.
Sulfated ash.Not more than 1.0 mg/g.
Hydriodic acid R1
HI. CAS Reg. No. 10034-85-2.
Procedure. Prepare by distilling hydriodic acid over red phosphorus, passing carbon dioxide R or nitrogen R through the apparatus during the distillation. Use the colourless or almost colourless, constant-boiling mixture (55 per cent to 58 per cent of HI) distilling between 126 °C and 127 °C.
Storage. Store at a dark place in small, amber glass-stoppered bottles previously flushed with carbon dioxide R or nitrogen R and sealed with paraffin.
Hydriodic acid (~970 g/L) TS
[hydriodic acid R] HI (SRIP, 1963, p. 95).
Hydrochloric acid (~0.365 g/L) TS
Hydrochloric acid (~250 g/L) TS, dilute with water to contain 0.365 g of HCl in 1000 mL.
Hydrochloric acid (~2.19 g/L) TS
Hydrochloric acid (~250 g/L) TS, dilute with water to contain 2.19 g of HCl in 1000 mL.
Hydrochloric acid (~3.65 g/L) TS
Hydrochloric acid (~250 g/L) TS, dilute with water to contain 3.65 g of HCl in 1000 mL.
Hydrochloric acid (~4 g/L) TS
Procedure.Dilute 10 mL of hydrochloric acid (~420 g/L) TS with sufficient water to produce 1000 mL (approximately 0.1 mol/L).
Hydrochloric acid (~10 g/L) TS
Hydrochloric acid (~250 g/L) TS, dilute with water to contain 10 g of HCl in 1000 mL.
Hydrochloric acid (~36.5 g/L) TS
Hydrochloric acid (~250 g/L) TS, dilute with water to contain 36.5 g of HCl in 1000 mL.
Hydrochloric acid (~70 g/L) TS
Procedure.Dilute 260 mL of hydrochloric acid (~250 g/L) TS with sufficient water to produce 1000 mL (approximately 2 mol/L); d~1.035.
Hydrochloric acid (~103 g/L) TS
Hydrochloric acid (~420 g/L) TS, dilute with water to contain 103 g of HCl in 1000 mL.
Hydrochloric acid (~146 g/L) TS
Procedure.Dilute hydrochloric acid (~250 g/L) TS with water to contain approximately 146 g of HCl in 1000 mL (approximately 4 mol/L).
Hydrochloric acid (~200 g/L) TS
Procedure. Dilute hydrochloric acid (~250 g/L) TS with water to contain approximately 200 g of HCl in 1000 mL (approximately 5.5 mol/L).
Hydrochloric acid (~200 g/L) TS
Hydrochloric acid (~420 g/L) TS, dilute with water to contain 200 g of HCl in 1000 mL.
Hydrochloric acid (~206 g/L) TS
Hydrochloric acid (~420 g/L) TS, dilute with water to contain 206 g of HCl in 1000 mL.
Hydrochloric acid (~250 g/L) AsTS
Hydrochloric acid (~250 g/L) TS that complies with the following tests A and B:
A. Dilute 10 mL with sufficient water to produce 50 mL, add 5 mL of ammonium thiocyanate (75 g/L) TS and stir immediately; no colour is produced.
B. To 50 mL add 0.2 mL of bromine AsTS, evaporate in a water-bath until reduced to 16 mL, adding more bromine AsTS if necessary to ensure that an excess, as indicated by the colour, is present throughout the evaporation. Add 50 mL of water and 5 drops of stannous chloride AsTS and apply the general test for arsenic. The colour of the stain produced is not more intense than that produced from a 0.2 mL standard stain showing that the amount of arsenic does not exceed 0.05 μg/mL.
Hydrochloric acid (~250 g/L) FeTS
Hydrochloric acid (~250 g/L) TS that complies with the following additional test: evaporate 5 mL nearly to dryness on a water-bath, add 40 mL of water, 2 mL of citric acid (180 g/L) FeTS and 2 drops of mercaptoacetic acid R; mix, make alkaline with ammonia (~100 g/L) FeTS and dilute to 50 mL with water; no pink colour is produced.
Hydrochloric acid (~250 g/L), stannated, AsTS
Procedure.Dilute 1 mL of stannous chloride AsTS with sufficient hydrochloric acid (~250 g/L) AsTS to produce 100 mL.
Hydrochloric acid (~330 g/L) TS
A solution of hydrochloric acid (~420 g/L) TS in water containing approximately 330 g of HCl per litre; d~1.15 (about 9 mol/L).
Hydrochloric acid (~420 g/L) TS
[hydrochloric acid, saturated, R] (SRIP, 1963, p. 96); d~1.18.
Hydrochloric acid (0.0001 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 3.647 mg of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under “Hydrochloric acid (1 mol/L) VS”.
Hydrochloric acid (0.005 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 0.1824 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid (0.01 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 0.3647 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid (0.015 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 0.5470 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid (0.02 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 0.7293 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/l) VS.
Hydrochloric acid (0.05 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 1.824 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 molLl) VS.
Hydrochloric acid (0.1 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 3.647 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid (0.2 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 7.293 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid (0.5 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 18.23 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid (1 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 36.47 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the 1 mol/L solution in the following manner: dissolve about 1.5 g, accurately weighed, of anhydrous sodium carbonate R, previously dried at 270 °C for 1 hour, in 50 mL of water and titrate with the hydrochloric acid solution using methyl orange/ethanol TS as indicator. Each 52.99 mg of anhydrous sodium carbonate is equivalent to 1 mL of hydrochloric acid (1 mol/L) VS.
Hydrochloric acid (2 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 72.93 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid (5 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with water to contain 182.35 g of HCl in 1000 mL.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid (~250 g/L) TS
A solution of hydrochloric acid (~420 g/L) TS in water containing approximately 250 g/L of HCl; d~1.12.
Hydrochloric acid ClTS
One millilitre contains 50 μg of Cl.
Procedure.Dilute 14.3 mL of hydrochloric acid (0.1 mol/L) VS with sufficient water to produce 1000 mL.
Hydrochloric acid, brominated, AsTS
Procedure.To 100 mL of hydrochloric acid (~250 g/L) AsTS add 1 mL of bromine AsTS.
Hydrochloric acid/ethanol (1mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with dehydrated ethanol R to contain 36.47 g of HCl in 1000 mL of dehydrated ethanol R.
Method of standardization. Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid/ethanol (0.1 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with dehydrated ethanol R to contain 3.647 g of HCl in 1000 mL of dehydrated ethanol R.
Method of standardization. Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid/methanol (0.01 mol/L) VS
Hydrochloric acid (~250 g/L) TS diluted with methanol R to contain 0.3647 g of HCl in 1000 mL of methanol R.
Method of standardization.Ascertain the exact concentration of the solution following the method described under hydrochloric acid (1 mol/L) VS.
Hydrochloric acid/methanol (0.1 mol/L) TS
Hydrochloric acid (~250 g/L) TS diluted with methanol R to contain 3.647 g of HCl in 1000 mL of methanol R.
Hydrocortisone R
C21H30O5. Use Hydrocortisone as described in the monograph for Hydrocortisone.
Hydrogen peroxide (~30 g/L) TS
A solution in water containing about 30 g of H2O2 per litre.
Hydrogen peroxide (~330 g/L) TS
[hydrogen peroxide (30% R] (SRIP, 1963, p. 97).
Hydrogen peroxide (~60 g/L) TS
A solution in water containing about 60 g of H2O2 per litre.
Hydrogen sulfide R
H2S (SRIP, 1963, p. 98).
Hydrogen sulfide TS
A saturated solution of hydrogen sulfide R in cold water.
Note:Hydrogen sulfide TS must be freshly prepared.
Hydroquinone R
C6H4(OH)2.
Description.Colourless or almost colourless crystals or a crystalline powder.
Solubility.Soluble in water, ethanol (~750 g/L) TS and ether R.
Melting temperature.About 173 °C.
Note.Hydroquinone R darkens on exposure to air and light.
Hydroxyethylcellulose R
Contains not less than 20% of C2H5O2, calculated with reference to the dried substance.
Description.A white or yellowish, flaky, heterogeneous mass; odourless.
Solubility.Practically insoluble in ethanol (~750 g/L) TS; after soaking for several hours in water, freely soluble in water.
Colour of solution.Transfer 2 g to a 200 mL glass-stoppered, conical flask, add 200 mL of carbon-dioxide-free water R, shake and allow to stand for 30 minutes. Repeat this operation until the substance has dissolved and filter through sintered glass. Observe 5 mL of the filtrate; it is colourless (keep the filtrate for the acidity or alkalinity test).
Loss on drying.To 1.0 g add 25 mL of water, stir and allow to stand. Repeat this operation until dissolved. Evaporate on a water-bath and dry to constant weight at 110 °C; it loses not more than 0.10 g/g. (Keep the dried substance for the assay.)
Acidity or alkalinity.To 10 mL of the filtrate obtained from the test for colour of solution add 2 drops of bromothymol blue/ethanol TS; a yellow colour is produced. Add 0.5 mL of potassium hydroxide (0.01 mol/L) VS; a green or blue solution is produced.
Assay.Place into a boiling flask as described under 2.9 Determination of methoxyl, 0.5 mL of acetic anhydride R, 0.05–0.10 g of phenol R, 0.20 g of red phosphorus R and 5.0 mL of hydriodic acid (~970 g/L) TS; connect the flask to the condenser, pass a slow, uniform stream of carbon dioxide R through the solution and heat for 60 minutes. Cool for 10 minutes and add 0.035 g, accurately weighed, of the dried substance obtained in the test for loss on drying. Proceed with this mixture as described under 2.9 Determination of methoxyl. For the calculation take an average of 3 determinations. Each mL of sodium thiosulfate (0.1 mol/L) VS is equivalent to 1.018 mg of C2H5O2.
Hydroxyethylcellulose TS
Procedure.Place 50 mL of water in a 100 mL beaker and add 2.0 g of hydroxyethylcellulose R. After 15 hours stir the solution for 1 minute and centrifuge for 15 minutes. Using a pipette separate 20 mL of the supernatant liquid.
Note:Hydroxyethylcellulose TS must be freshly prepared.
Hydroxylamine hydrochloride (200 g/L) TS
A solution of hydroxylamine hydrochloride R containing about 200 g of NH2OH,HCl per litre.
Hydroxylamine hydrochloride (70 g/L) TS
Procedure.Dissolve 69.5 g of hydroxylamine hydrochloride R in sufficient water to produce 1000 mL (1 mol/L).
Hydroxylamine hydrochloride R
NH2OH,HCl (SRIP, 1963, p. 99).
Hydroxylamine hydrochloride TS
Procedure.Dissolve 1 g of hydroxylamine hydrochloride R in 50 mL of water and add 50 mL of ethanol (~750 g/L) TS and 1 mL of bromophenol blue/ethanol TS; then add sodium hydroxide (0.1 mol/L) VS until the solution becomes green.
Hydroxylamine hydrochloride TS2
Procedure. Dissolve 3.5 g of hydroxylamine hydrochloride R in 95 mL of ethanol (~535 g/L) TS, add 0.5 mL of bromophenol blue (1 g/L) TS and sufficient potassium hydroxide/ethanol (0.5 mol/L) TS until a greenish tint is developed.
Dilute the solution to 100 mL with ethanol (~535 g/L) TS.
8-Hydroxyquinoline R
8-Quinolinol; C9H7NO.
Description.A white to yellowish white, crystalline powder.
Solubility.Practically insoluble in water and ether R; freely soluble in ethanol (~750 g/L) TS and acetone R.
Melting point.About 74 °C.
8-Hydroxyquinoline/chloroform TS
Procedure.Dissolve 1 g of 8-hydroxyquinoline R in sufficient chloroform R to produce 100 mL.
Hypophosphorous acid R
Phosphinic acid; H3PO2 (SRIP, 1963, p. 100).
Hypophosphorous acid, dilute, TS
A solution of hypophosphorous acid R containing about 100 g of H3PO2 per 1000 mL.
Hypoxanthine R
1,7-dihydro-6H-purin-6-one; C5H4N4O.
A commercially available reagent of suitable grade.
Description. A white, crystalline powder.
Solubility. Very slightly soluble in water, sparingly soluble in boiling water, soluble in dilute acids and in dilute alkali hydroxide solutions.
Melting point. Decomposes without melting at about 150 °C.
Thin-Layer Chromatography. Examine as prescribed in the monograph on Mercaptopurine; the chromatogram shows only one principal spot.