Supplementary information: Test methods used for investigative testing of suspicious samples: Tests for diethylene glycol and ethylene glycol in liquid preparations for oral use
Introduction and scope
Diethylene glycol (DEG) and ethylene glycol (EG) are toxic substances used as industrial solvents and antifreeze agents that can be fatal, even taken in small amounts, in particular for children.
The minimum safe levels for humans for ingested DEG and EG are not known with certainty but it is generally recognised that a detection level of 0.10% for each substance is considered adequate for screening raw materials and finished products for the substances from a safety point of view.
A suitable and widely used analytical technique to test pharmaceutical products precisely and accurately for DEG and EG is gas chromatography (GC).
For National Quality Control Laboratories(NQCLs) without access to this analysis technique, a screening for non-compliance method by thin-layer chromatography (TLC) is described, which allows the detection of DEG or EGconcentrations down to about 0.2% (m/m) (see Screening for non-compliance by thin-layer chromatography). As the exact detection limits may vary from laboratory to laboratory, analysts shall verify the value when performing the method.
It is not possible to distinguish between DEG and EG as both contaminants co-elute in the described TLC procedure. In performing the test, analysts will determine the combined concentration of DEG and EG rather than the concentrations of the individual contaminants. Consequently, reference solutions of a 50:50 (m/m) mixture of DEG and EG are used for the calibration of the analyte response.
Considering the uncertainty of the semi-quantitative TLC method, the subjective element in the determination of semi-quantitative test results and the potential for the detection limit to be above the 0.1% limit that is considered safe, there remains the risk of missing detection of some finished products having individual concentrations of DEG or EG above 0.10% but below the detection limit. For example, with a limit of detection of 0.5%, a sample with 0.3% DEG and no EG would give a false negative result.
While upstream screening using the TLC method enables less resourced NQCLs to increase the chance of detecting samples and identifying adulterated products that pose a risk for patients, laboratories wishing to apply the screening method shall consider approaches for reducing the risk of false negative results, such as subsequently confirming results at collaborating laboratories or regional centres using GC (see Confirmatory testing by gas chromatography).
NQCLs with access to GC are advised to use the procedure "Confirmatory testing by gas chromatography" without the upstream screening for non-compliance.
The GC method is more sensitive than the TLC procedure by at least a factor of about two. Concentrations of DEG or EG down to 0.10% (m/m) can be determined by GC. The method can be adapted to also analyse samples of excipients used in the manufacture of pharmaceutical preparations.
Laboratories whose investigations have confirmed DEG or EG contaminations in excipients or finished products shall inform the responsible regulatory authorities without delay.
For the definitions of reagents (R) and test solutions (TS), please consult The International Pharmacopoeia. This document contains images to illustrate certain steps of the analytical procedures (see Images).
Screening for non-compliance by thin-layer chromatography
Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using a plate coated with silica gel R5 or R6 and of a suitable size (e.g. 10 cm x 10 cm, 10 cm x 20 cm or 20 cm x 20 cm).
Saturate a chromatographic tank of a suitable size by lining its walls with filter paper. Pour a sufficient quantity of a freshly prepared mixture of toluene R, acetone R and ammonia (~10 g/L) TS(5:85:10 V/V/V) into the chromatographic tank. After impregnation of the filter paper, the mobile phase shall form a layer of appropriate depth, usually 1 cm. Replace the lid and allow the tank to stand for 1 hour at 20-25 °C to complete the saturation of the tank.
Prepare the following solutions, using methanol R as the diluent.
For solution (A) (sample solution, 100 mg sample/mL), transfer 1.000 g of the liquid preparation for oral use under investigation to a 10.0 mL volumetric flask. Add 5 mL of the diluent, stopper the flask and shake vigorously. Make up to volume with the diluent and mix thoroughly. (Incomplete mixing of sample and diluent can lead to an underestimation of DEG/EG in the sample.)
For solution (B) (EG/DEG reference solution, 20% (m/m), 20 mg EG/DEG/mL), dissolve 500 mg of diethylene glycol R and 500 mg of ethylene glycol in 50.0 mL of diluent.
For solution (C) (EG/DEG reference solution, 10% (m/m), 10 mg EG/DEG /mL), dilute 10.0 mL of solution (B) to 20.0 mL with diluent.
For solution (D) (EG/DEG reference solution, 1% (m/m), 1 mg EG/DEG /mL), dilute 5.0 mL of solution (B) to 100.0 mL with diluent.
For solution (E) (EG/DEG reference solution, 0.5% (m/m), 0.5 mg EG/DEG /mL), dilute 2.5 mL of solution (B) to 100.0 mL with diluent.
For solution (F) (EG/DEG reference solution, 0.2% (m/m), 0.2 mg EG/DEG /mL), dilute 1.0 mL of solution (B) to 100.0 mL with diluent.
If information on the excipients in the liquid preparation for oral use under investigation is available, prepare in addition solution (G) by dissolving a suitable amount of each of the excipients in a suitable volume of diluent and filtering, if needed.
For the visualization solution, prepare a mixture of 0.75 g of potassium permanganate R, 5 g of sodium carbonate R and 0.625 mL of sodium hydroxide (~ 100 g/L) TS in 100 mL of water R.
Apply separately to the plate, 2 µL of each of solutions (A), (B), (C), (D), (E), (F) and, if available, (G) at a distance of about 2 cm from the lower edge and from the sides of the plate. Apply the solutions on a line parallel to the lower edge, allowing the standard solutions to bracket the sample solution. Use a device that allows the accurate and reproducible dispensing of the prescribed volume onto the plate (e.g. micro capillary pipettes).
Apply the solutions in sufficiently small portions to obtain circular spots of 2-5 mm in diameter, or lines, if appropriate. Allow an interval of at least 10 mm between the centres of circular spots or between the lines.
When the solvent of the applied solutions has evaporated, place the plate in the chromatographic tank, ensuring that the plate is as vertical as possible and that the spots are above the surface of the mobile phase. Close the chromatographic tank and maintain it at 20-25 °C, protected from sunlight and placed in a draught-free area. Remove the plate when the mobile phase has moved over 75% of the distance measured between the points of application and the top of the plate.
Dry the plate and spray it with the visualization solution (see Image 1) until a fully covered purple background is obtained (see Image 2 ). Dry the plate using a hairdryer, applying heat uniformly across the plate while maintaining a distance of approximately 10 cm (see Image 3). Continue heating the plate until bright yellow spots are visible.
The test is not valid unless the chromatogram obtained with solution (E) shows a spot due to EG/DEG. Determine the detection limit by evaluating the spots due to EG/DEG in the chromatograms obtained with solutions (D), (E) and (F).
Should the chromatogram obtained with solution (E) fail to show a spot due to EG/DEG, consider increasing the volume of sample and standard solutions applied to the plate.
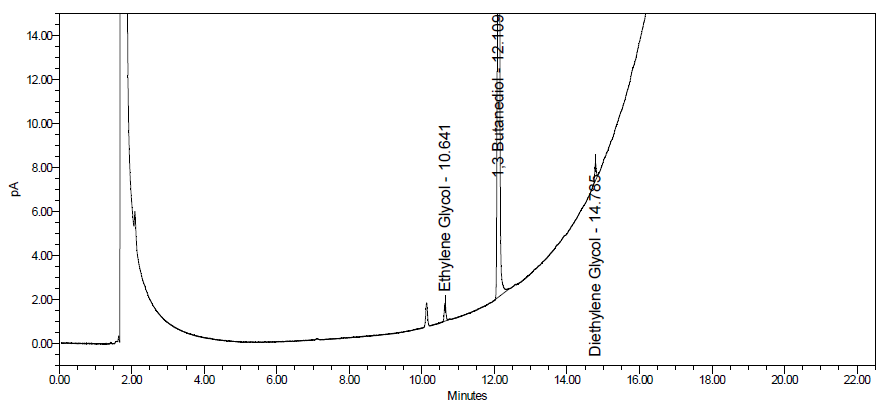
In the chromatogram obtained with solution (A), the following substances, if present, are eluted with the following Rf values: sorbitol about 0.10; glycerol about 0.41; DEG and EG about 0.54; propylene glycol about 0.62. Use the chromatogram obtained with solution (G) to identify further spots due to excipients. For typical chromatograms obtained for EG/DEG reference solutions and samples, see Image 4.
Identify the spots due to EG/DEG in the chromatograms obtained and estimate the approximate combined percentage content of DEG and EG (m/m) in the liquid preparation for oral use under investigation by comparing the intensity of the spot due to EG/DEG in the chromatogram obtained with solution (A), if present, with the intensities of the spots due to EG/DEG in the chromatograms obtained with solutions (B) to (F).
Confirmatory testing by gas chromatography
Carry out the test as described under 1.14.1 Chromatography, Gas chromatography using the internal standard method.
For the procedure, use a capillary glass or quartz column (30 m × 0.53 mm), the inner surface of which is coated with a thick layer of macrogol 20M R (1.0 μm). Maintain the temperature of the column at 100 °C for 5 minutes. Increase the temperature at a rate of 10 °C per minute to 245 °C and maintain it at this point for 4 minutes. Maintain the temperature of the injection port and the detector at 250 °C. Use helium R as the carrier gas with a linear velocity of about 38 cm per second. Use split injection ratio of 1:20 and a flame-ionization detector for detection.
Laboratories that can only obtain a column with a maximum temperature of 240 °C or below may change the temperature accordingly, if necessary. However, lowering the temperature may affect the ability of the method to clean the column of substances with a high boiling point thus deteriorating column performance over time.
The described procedure was validated using helium as a carrier gas. The use of nitrogen or hydrogen may also be suitable with little or no changes in peak resolution. Helium is a non-renewable gas that may be or eventually become difficult to source. Nitrogen and hydrogen can be produced on-demand using suitable generators.
For solution (IS)(10 mg IS per mL), weigh 0.500 g of the internal standard 1,3-butanediol R and dilute to 50.0 mL with water R.
During the elaboration of the analytical procedure, it was noted that the peak due to 1,3-butanediol may show peak tailing when columns with thinner layers of macrogol are used. In such cases, 1,3 propanediol Rwas found to be a suitable alternative to 1,3-butanediol.
For solution (A)(sample solution, 25 mg of sample per mL), weigh 0.500 g of the liquid preparation for oral use under investigation into a 20 mL volumetric flask, add 1.0 mL of solution (IS) and 1.0 mL of water R and mix thoroughly. Make up to volume with ethanol R and sonicate for 5 minutes. Place the solution in an ice bath for 15 minutes and filter.
For solution (B)(sample solution without IS), weigh 0.500 g of the liquid preparation for oral solution under investigation into a 20 mL volumetric flask, add 2.0 mL of water R and mix thoroughly. Make up to volume with ethanol R and sonicate for 5 minutes. Place the solution in an ice bath for 15 minutes and filter.
For solution (C)(EG, PG, DEG stock solution; 10 mg each per mL), weigh 1.000 g of each ethylene glycol RS, propylene glycol R and diethylene glycol RS and dilute to 100.0 mL with ethanol R.
For solution (D)(EG, PG, DEG stock solution; 1.0 mg each per mL) dilute 2.0 mL of solution (C) to 20.0 mL with ethanol R.
For solution (E)(EG, DEG calibration solution, 1.25 mg each per mL, 5% with regards to sample concentration in solution (A)), mix 25.0 mL of solution (C) with 10.0 mL of solution (IS), 15 mL of water R and dilute to 200.0 mL with ethanol R.
For solution (F) (EG, DEG calibration solution, 0.5 mg each per mL, 2% with regards to sample concentration in solution (A)), mix 1.0 mL of solution (C) with 1.0 mL of solution (IS), 1.5 mL of water R and dilute to 20.0 mL with ethanol R.
For solution (G)(EG, DEG calibration solution, 0.1 mg each per mL, 0.4%with regards to sample concentration in solution (A)), mix 2.0 mL of solution (D) with 1.0 mL of solution (IS), 1.5 mL of water R and dilute to 20.0 mL with ethanol R.
For solution (H)(EG, DEG calibration solution, 0.025 mg each per mL, 0.1% with regards to sample concentration in solution (A)), dilute 2.0 mL of solution (D) to 20.0 mL with ethanol R. Mix 5.0 mL of this solution with 1.0 mL solution (IS), 1.5 mL of water R and dilute to 20.0 mL with ethanol R.
For solution (I)(sensitivity control solution, 0.01 mg each per mL), dilute 2.0 mL of solution (D) to 20.0 mL with ethanol R. Mix 2.0 mL of this solution with 1.0 mL solution (IS), 1.5 mL of water R and dilute to 20.0 mL with ethanol R.
Inject separately 1.0 µl each of solutions (A), (B), (E), (F), (G), (H) and (I) and record the chromatograms. After each injection, wash the needle first with water R, then with methanol / water (1:1 V/V) and finally with 2-propanol R.
Depending on the dimensions of the liner used, the injection volume may need to be adjusted to avoid backflushing of the sample.
Measure the response of the peaks corresponding to DEG, EG, propylene glycol and 1,3-butanediol in the chromatograms obtained. The substances, if present, are eluted at the following relative retention with reference to 1,3-butanediol (retention time about 14 minutes): propylene glycol about 0.83; EG about 0.87; and DEG about 1.20.
The test is not valid unless the resolution between the peaks corresponding to EG and propylene glycol in the chromatogram obtained with solution (G), is at least 4.0. For a typical chromatogram obtained for solution (G), see Image 5.
Also, the test is not valid unless, in the chromatogram obtained with solution (I), the signal-to-noise ratios of the peaks due to DEG and EG are both at least 10. For a typical chromatogram obtained for solution (G), see Image 6.
Furthermore, the test is not valid unless, in the chromatogram obtained with solution (B), any peak having the same retention time as 1,3-butanediol is not more than 0.01 times the area of the peak corresponding to 1,3-butanediol in the chromatogram obtained with solution (A).
The procedure was successfully applied to numerous samples from the market. However, it cannot be fully excluded that certain excipients or components of samples may interfere with the peaks due to EG, DEG or the internal standard. In such cases, the use of a mass-selective detector may enhance the selectivity of the analyte response.
Calculate the ratios between the responses of the peaks due to EG/DEG and the responses of the peak due to 1,3-butanediol in the chromatograms obtained with the solutions (A), (E), (F), (G) and (H).
Determine a separate calibration function each for EG and DEG by plotting the ratios obtained for solutions (E), (F), (G) and (H) on the ordinate against the percentage content of EG/DEG reference substances with regards to the sample concentration in solution (A) on the abscissa. Consider the accurate weights of the reference substances, the accurate weight of the sample and the declared content of C2H6O2 or C4H10O3 in ethylene glycol RS and diethylene glycol RS.
Use the ratio obtained for solution (A) and the calibration functions to calculate the percentage content (m/m) of DEG and EG in the liquid preparation for oral use. If one or both results obtained are above 5%, prepare solutions (A) and (B) using a lower weight of liquid preparation for oral use under investigation and repeat the analysis to ensure that the obtained result(s) lies within the calibration range. For samples containing more than 15%, prepare an intermediate dilution in water R and use this solution to prepare solutions (A) and (B).
The percentage concentrations (m/m) of DEG and EG in the liquid preparation for oral use are each not more than 0.10%.
Image 1. Spray the developed plate with the visualization solution using an atomizer.

Image 2. Spray the plate with the visualization solution until a fully covered purple background is obtained.

Image 3. Dry the plate using a hairdryer, applying heat uniformly across the plate while maintaining a distance of approximately 10 cm. Continue heating the plate until bright yellow spots are visible.

Image 4. Typical chromatograms obtained with EG/DEG reference solutions and samples (without DEG/EG and spiked with 1% DEG/EG).
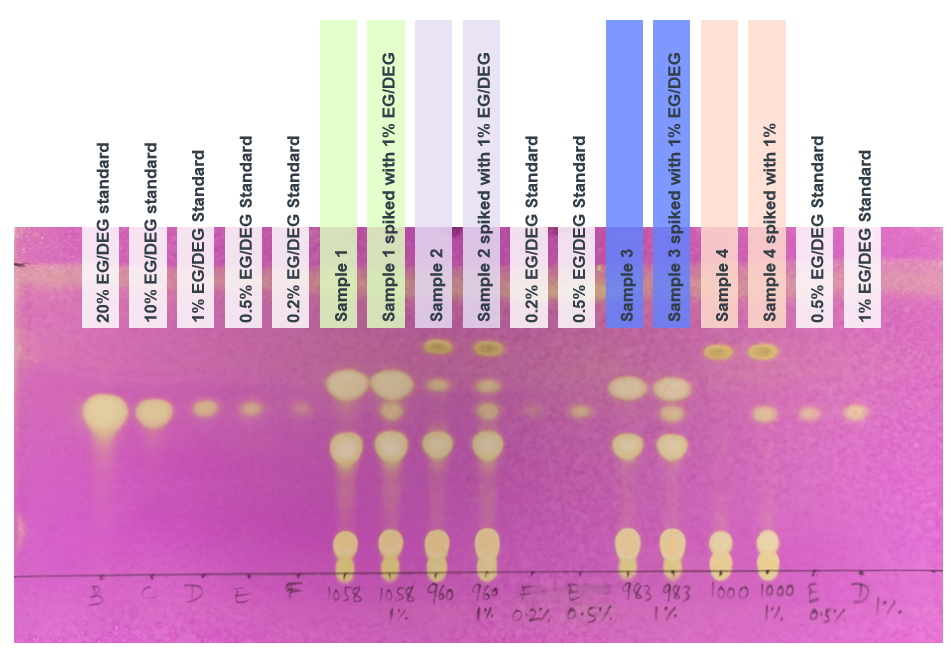
Image 5. Typical chromatogram obtained with solution (G).
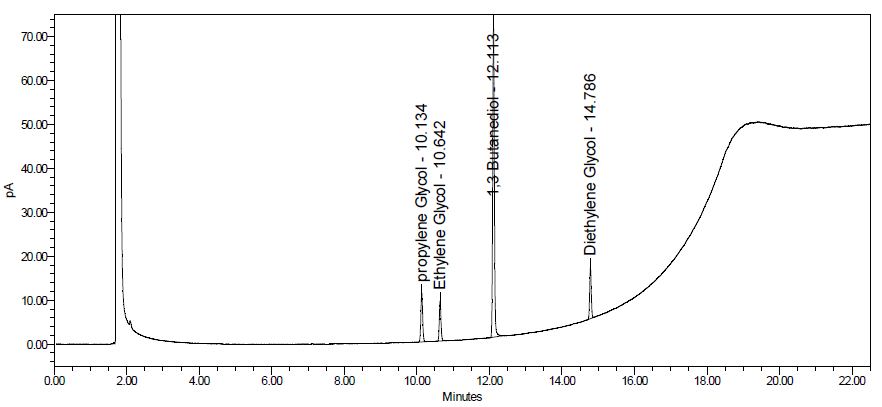
Image 6. Typical chromatogram obtained with solution (I).