Monographs: Pharmaceutical substances: Doxorubicin hydrochloride (Doxorubicini hydrochloridum)
Molecular formula. C27H29NO11,HCl
Relative molecular mass. 580.0
Graphic formula.
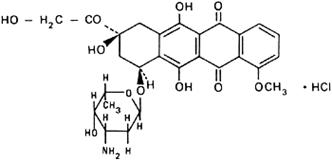
Chemical name. (8S,10S)-10-[(3-Amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione hydrochloride; (8S-cis)-10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione hydrochloride; CAS Reg. No. 25316-40-9.
Description. A red-orange, crystalline powder.
Solubility. Soluble in water and methanol R; practically insoluble in ether R.
Category. Cytotoxic drug.
Storage. Doxorubicin hydrochloride should be kept in a tightly closed container.
Additional information. Doxorubicin hydrochloride is hygroscopic; it is poisonous. CAUTION: Doxorubicin hydrochloride must be handled with care, avoiding contact with the skin and inhalation of airborne particles.
Requirements
Definition. Doxorubicin hydrochloride contains not less than 97.0% and not more than 102.0% of C27H29NO11,HCl, calculated with reference to the anhydrous substance.
Identity tests
A. The absorption spectrum of a 20 μg/mL solution in methanol R, when observed between 220 nm and 600 nm, is qualitatively similar to that of a 20 μg/mL solution of doxorubicin hydrochloride RS in methanol R (maxima occur at about 233 nm, 253 nm, 290 nm, 477 nm, 495 nm and 530 nm; minima occur at about 245 nm, 280 nm and 350 nm). The absorbances of the solutions at the respective maxima do not differ from each other by more than 3%. The absorbance of a 1-cm layer at the wavelengths of the main maxima are about 1.32, 0.88, 0.30, 0.46, 0.44 and 0.24, respectively.
B. See the test described below under "Related substances". The principal spot obtained with solution A corresponds in position, appearance and intensity with that obtained with solution B.
C. Dissolve 2 mg in 2 mL of methanol R, add 2 mL of water and 0.05 mL of sodium hydroxide (~80 g/l) TS; the orange-red colour of the solution turns to blue-violet.
D. A 0.05 g/mL solution yields reaction B described under 2.1 General identification tests as characteristic of chlorides.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A, using about 0.25 g of the substance; the water content is not more than 40 mg/g.
pH value. pH of a 5.0 mg/mL solution, 3.8-6.5.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance and a mixture of 80 volumes of chloroform R, 20 volumes of methanol R and 5 volumes of glacial acetic acid R as the mobile phase. Apply separately to the plate 10 μl of each of 4 solutions in methanol R containing (A) 2.0 mg of the test substance per mL, (B) 2.0 mg of doxorubicin hydrochloride RS per mL, (C) 20 mg of the test substance per mL, and (D) 0.40 mg of the test substance per mL. After removing the plate from the chromatographic chamber, allow it to dry in air and examine the chromatogram in daylight. Any spot obtained with solution C, other than the principal spot, is not more intense than that obtained with solution D.
Assay. Dissolve about 20 mg, accurately weighed, in sufficient methanol R to produce 100 mL; dilute 10 mL of this solution to 100 mL with the same solvent. Measure the absorbance of a 1-cm layer of the diluted solution at the maximum at about 495 nm. Calculate the amount of C27H29NO11,HCl in the substance being tested by comparison with doxorubicin hydrochloride RS, similarly and concurrently examined. In an adequately calibrated spectrophotometer the absorbance of the reference solution should be 0.44 ± 0.02.