Monographs: Pharmaceutical substances: Doxycycline hyclate (Doxycyclini hyclas)
Molecular formula. C22H24N2O8, HCl, ½ C2H6O, ½ H2O
Relative molecular mass. 512.9
Graphic formula
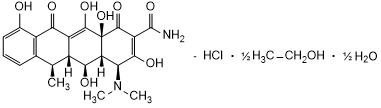
Chemical name. (4S,4aR,5S,5aR,6R,12a S )-4-(Dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide hydrochloride, compound with ethanol (1:0.5), hemihydrate; CAS Reg. No. 24390-14-5.
Description. A yellow, crystalline powder.
Solubility. Freely soluble in water R and in methanol R. It dissolves in solutions of alkali hydroxides and carbonates.
Category. Antibacterial and antimalarial.
Storage. Doxycycline hyclate should be kept in a tightly closed container, protected from light.
Additional information. Even in the absence of light, Doxycycline hyclate is gradually degraded on exposure to a humid atmosphere, the decomposition being faster at higher temperatures.
Requirements
Definition. Doxycycline hyclate contains not less than 95.0% and not more than 102.0% of C22H24N2O8,HCl, calculated with reference to the anhydrous and ethanol-free substance.
Identity tests
- Either tests A, B and D or tests C and D may be applied.
-
Dissolve 5 mg in 2 mL of sulfuric acid (~1760 g/L) TS; an intense yellow colour is produced.
-
Dissolve 5 mg in 2.0 mL of water and add 0.05 mL of ferric chloride (~25 g/L) TS; a dark red-brown colour is produced.
-
See the method described below under Assay. The retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).
-
A 20 mg/mL solution yields reaction B described under 2.1 General identification tests as characteristic of chlorides.
Sulfated ash (2.3). Not more than 4.0 mg/g.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method , Method A, using about 1.2 g of the test substance; the water content is not less than 14 mg/g and not more than 28 mg/g.
Ethanol. Carry out the test as described under 1.14.1 Chromatography, Gas chromatography . Use a fused-silica capillary column, 50 meters long, 0.32 mm in internal diameter coated with a poly(dimethyl)siloxane R (film thickness of 5 µm).
As a detector, use a flame ionization detector. Maintain the temperature of the column at 70 °C for 7 minutes, then raise the temperature at a rate of 15 °C per minute to 160 °C and maintain the temperature for 2 minutes. Keep the temperature of the injection port at 200 °C and that of the flame ionization detector at 250 °C.
Use helium R as the carrier gas with a linear velocity of about 37 cm per second and a split ratio of 1:10.
Prepare the following solutions R. For the internal standard solution, dilute 0.50 mL of 1-propanol R to 1.0 L with water R. For solution (1), dissolve 0.10 g of the test substance in the internal standard solution and dilute to 10.0 mL with the same solvent. For solution (2), dilute 0.50 mL of dehydrated ethanol R to 100.0 mL with the internal standard solution. Dilute 1.0 mL of this solution to 10.0 mL with the internal standard solution.
Inject alternately 1 μL each of solutions (1) and (2).
In the chromatogram obtained with solution (1), ethanol is eluted with a relative retention of about 0.6 with reference to 1-propanol (retention time about 7 minutes).
Measure the peak responses of ethanol and of the internal standard 1-propanol. Calculate the percentage content of ethanol using the ratios of the responses of ethanol to the responses of the internal standard, taking the weight per mL of ethanol at 20 °C to be 0.790 g; not less than 43 mg/g and not more than 60 mg/g.
pH value (1.13). pH of a 10 mg/mL solution, 2.0-3.0.
Light-absorbing impurities. Prepare a 10 mg/mL solution in a mixture of 1 volume of hydrochloric acid (1 mol/L) VS and 99 volumes of methanol R and measure the absorbance (1.6) of a 1-cm layer at 490 nm; the absorbance does not exceed 0.07 for the anhydrous and ethanol-free substance.
Related substances. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using the chromatographic conditions and preparing the solutions as described under assay.
Inject 20 μL of solution (5). The test is not valid unless the resolution between the first peak (metacycline) and the second peak (6-epidoxycycline) is greater than 1.25 and the resolution between the second peak and the third peak (doxycycline) is greater than 2.0. If necessary, adjust the tert-butanol R content in the mobile phase to obtain a long retention time for doxycycline and to improve the separation of doxycycline and related substances. The test is not valid unless the symmetry factor for the third peak (doxycycline) is less than 1.25.
In the chromatogram obtained with solution (1), the area of any peak corresponding to metacycline or to 6-epidoxycycline is not greater than the area of the corresponding peak in the chromatogram obtained with solution (6) (2%), the area of any peak appearing between the solvent peak and the peak corresponding to metacycline and the area of any peak appearing on the tail of the main peak is not greater than 0.25 times the area of the peak corresponding to 6-epidoxycycline in the chromatogram obtained with solution (6) (0.5% ).
Assay. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography , using a stainless steel column (25 cm × 4.6 mm) packed with particles of styrene-divinylbenzene copolymer (8-10 μm). As the mobile phase, use a solution prepared as follows: transfer 60.0 g of tert-butanol R with the aid of 200 mL of water to a 1000 mL volumetric flask. Add 400 mL of phosphate buffer, pH 8.0 (0.05 mol/L), TS, 50 mL of a solution of 10 mg of tetrabutylammonium hydrogen sulfate R per mL adjusted to pH 8.0 with sodium hydroxide (~80 g/L) TS, and 20 mL of sodium edetate (20 g/L) TS adjusted to pH 8.0 with sodium hydroxide (~80 g/L) TS. Dilute to 1000 mL with water R.
Prepare the following solutions in hydrochloric acid (0.01 mol/L) VS immediately before use. Solution (1) contains 0.80 mg of the test substance, solution (2) contains 0.80 mg of doxycycline hyclate RS per mL, solution (3) 0.80 mg of 6-epidoxycycline hydrochloride RS per mL, solution (4) 0.80 mg of metacycline hydrochloride RS per mL. For solution (5), mix 4.0 mL of solution (2) with 1.5 mL of solution (3) and 1.0 mL of solution (4), and dilute to 25 mL with hydrochloric acid (0.01 mol/L) VS. For solution (6), mix 2.0 mL of solution (3) and 2.0 mL of solution (4) and dilute to 100 mL with hydrochloric acid (0.01 mol/L) VS.
Operate with a flow rate of about 0.9 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of about 254 nm. Maintain the temperature of the column at 60°C.
Inject 20 μL of solution (5). The test is not valid unless the resolution between the first peak (metacycline) and the second peak (6-epidoxycycline) is greater than 1.25 and the resolution between the second peak and the third peak (doxycycline) is greater than 2.0. If necessary, adjust the tert-butanol R content in the mobile phase. The test is not valid unless the symmetry factor for the third peak is less than 1.25.
Inject alternately 20 μL each of solutions (1) and (2).
Measure the areas of the peak responses obtained in the chromatograms from solutions (1) and (2) and calculate the content of C22H24N2O8,HCl, taking into account the declared content of C22H24N2O8,HCl in doxycycline hyclate RS.
Impurities. The impurities limited by the requirements of this monograph include:
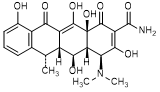
A. (4S,4aR,5S,5aR,6S,12aS)-4-(Dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (6-epidoxycycline),
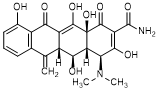
B. (4S,4aR,5S,5aR,12aS)-4-(Dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methylidene-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (metacycline),
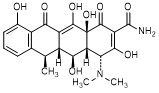
C. (4R,4aR,5S,5aR,6R,12aS)-4-(Dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (4-epidoxycycline),
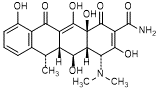
D. (4R,4aR,5S,5aR,6S,12aS)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (4-epi-6-epidoxycycline).
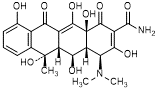
E. (4S,4aR,5R,5aR,6S,12aS)-4-(dimethylamino)-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (oxytetracycline).
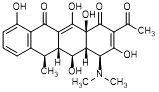
F. (4S,4aR,5S,5aR,6R,12aS)-2-acetyl-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-4a,5a,6,12a-tetrahydrotetracene-1,11(4H,5H)-dione (2-acetyl-2-decarbamoyldoxycycline).