Monographs: Pharmaceutical substances: Hydralazine hydrochloride (Hydralazini hydrochloridum)
Molecular formula. C8H8N4,HCl
Relative molecular mass. 196.6
Graphic formula.
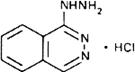
Chemical name. 1-Hydrazinophthalazine monohydrochloride; 1(2H)-phthalazinone hydrazone monohydrochloride; CAS Reg. No. 304-20-1.
Other name. Apressinum.
Description. A white or almost white, crystalline powder; odourless.
Solubility. Soluble in 25 parts of water; slightly soluble in ethanol (~750 g/l) TS; very slightly soluble in ether R.
Category. Antihypertensive drug.
Storage. Hydralazine hydrochloride should be kept in a well-closed container, protected from light.
Additional information. Hydralazine hydrochloride melts at about 275 °C with decomposition. Even in the absence of light, it is gradually degraded on exposure to a humid atmosphere, the decomposition being faster at higher temperatures.
Requirements
Definition. Hydralazine hydrochloride contains not less than 98.0% and not more than 101.0% of C8H8N4,HCl, calculated with reference to the dried substance.
Identity tests
A. The absorption spectrum of a 10 μg/mL solution, when observed between 220 nm and 350 nm, exhibits maxima at 240 nm, 260 nm, 303 nm, and 315 nm; the absorbances of a 1-cm layer at these wavelenghts are about 0.58, 0.54, 0.27, and 0.21, respectively.
B. Dissolve 0.5 g in a mixture of 100 mL of water and 8 mL of hydrochloric acid (~70 g/l) TS, add 20 mL of sodium nitrite (10 g/l) TS, allow to stand for 10 minutes and filter. Wash the residue with water and dry at 105°C; melting temperature, about 210°C.
C. A 20 mg/mL solution yields reaction A described under 2.1 General identification tests as characteristic of chlorides.
Water-insoluble substances. Transfer 2.0 g to a 250-mL conical flask, add 100 mL of water, and shake by mechanical means for 30 minutes. Filter the solution through a tared sintered glass crucible, rinse the flask, and wash any undissolved residue into the crucible. Wash the residue with three 10-mL portions of water, dry at 105°C for 3 hours, cool and weigh; the residue weighs not more than 10 mg (5 mg/g).
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry at ambient temperature under reduced pressure (not exceeding 0.6 kPa or about 5 mm of mercury) over phosphorus pentoxide R for 8 hours; it loses not more than 5.0 mg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance. For the mobile phase, shake a mixture of 2 volumes of ethyl acetate R, 2 volumes of ammonia (~260 g/l) TS and 8 volumes of hexane R, allow to separate and use the upper layer. For the preparation of the test solution dissolve 0.10 g of the test substance in a mixture of 100 volumes of methanol R and 1 volume of hydrochloric acid (~420 g/l) TS, and dilute to 20 mL with the same solvent mixture; to 2.0 mL of this solution add 1.0 mL of salicylaldehyde TS, centrifuge and decant the supernatant liquid (solution A). For the reference solution, dissolve 25.0 mg of hydrazine sulfate R in 10 mL of water and dilute to 100 mL using a mixture of 1 volume of hydrochloric acid (~420 g/l) TS and 100 volumes of methanol R; dilute 1.0 mL to 100 mL with the same solvent mixture. To 2.0 mL of this solution add 1.0 mL of salicylaldehyde TS, centrifuge and decant the supernatant liquid (solution B). Apply separately to the plate 40 μl of each of solutions A and B. After removing the plate from the chromatographic chamber, allow it to dry in air and spray with 4-dimethylaminobenzaldehyde TS6. Examine the chromatogram in ultraviolet light (254 nm). Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Assay. Dissolve about 0.15 g, accurately weighed, in 25 mL of water, add 25 mL of hydrochloric acid (~420 g/l) TS, cool to room temperature, add 5 mL of chloroform R, and titrate with potassium iodate (0.05 mol/l) VS, shaking continuously, until the purple colour of iodine in the chloroform layer disappears. The end-point is reached when the chloroform layer remains colourless for at least 5 minutes. Each mL of potassium iodate (0.05 mol/l) VS is equivalent to 9.832 mg of C8H8N4,HCl.