Monographs: Pharmaceutical substances: Levothyroxine sodium (Levothyroxinum natricum)
Molecular formula. C15H10I4NNaO4 (anhydrous); C15H10I4NNaO4,H2O (mono-hydrate).
Relative molecular mass. 798.9 (anhydrous); 816.9 (monohydrate).
Graphic formula.
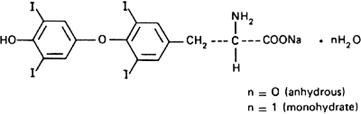
Chemical name. Monosodium L-thyroxine; monosodium O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-L-tyrosine; 3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]-L-alanine monosodium salt; CAS Reg. No. 55-03-8 (anhydrous); Monosodium L-thyroxine monohydrate; monosodium O-(4-hydroxy-3,5-diiodo-phenyl)-3,5-diiodo-L-tyrosine monohydrate; 3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]-L-alanine monosodium salt monohydrate; CAS Reg. No. 31178-59-3 (monohydrate).
Other name. Thyroxine sodium.
Description. An almost white or slightly coloured powder, or a fine, slightly coloured, crystalline powder; odourless.
Solubility. Very slightly soluble in water; slightly soluble in ethanol (~750 g/L) TS; practically insoluble in acetone R and ether R. It dissolves in solutions of alkali hydroxides.
Category. Thyroid hormone.
Storage. Levothyroxine sodium should be kept in a tightly closed container, protected from light.
Additional information. Levothyroxine sodium may contain a variable quantity of water of crystallization; anhydrous levothyroxine sodium is hygroscopic.
Requirements
Definition. Levothyroxine sodium contains not less than 97.0% and not more than 101.0% of C15H10I4NNaO4, calculated with reference to the dried substance.
Identity tests
A. Dissolve 5 mg in 2.0 mL of nitric acid (~130 g/L) TS and warm the solution; a brown to violet colour is produced. Cool, add 1.0 mL of chloroform R and shake; the colour of the chloroform layer turns violet.
B. Dissolve 5 mg in a mixture of 2.0 mL of ethanol (~750 g/L) TS and about 0.2 mL of hydrochloric acid (~70 g/L) TS, add about 0.25 mL of sodium nitrite (10 g/L) TS and allow to stand for 15 minutes or heat for 2–3 minutes in a water-bath; a yellow solution is produced. Cool and add ammonia (~100 g/L) TS to make the solution alkaline; the colour changes to red.
C. Examine the chromatograms obtained in the test for liothyronine (see below). The principal spot in the chromatogram obtained with solution A is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution B.
D. When tested for sodium as described under 2.1 General identification tests, yields the characteristic reactions. If reaction B is to be used ignite 20 mg and dissolve the residue in acetic acid (~60 g/L) TS.
Specific optical rotation. Dissolve 0.5 g in 22 mL of a gently boiling mixture of 1 volume of hydrochloric acid (1 mol/L) VS and 4 volumes of ethanol (~750 g/L) TS. Cool and dilute to 25 mL with the same mixture of solvents. Calculate with reference to the dried substance;  = +16.0° to +20.0°.
= +16.0° to +20.0°.
Loss on drying. Dry to constant weight at 105 °C; it loses not less than 60 mg/g and not more than 120 mg/g.
Liothyronine. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using a plate coated with a mixture of 30 g of silica gel R3 and 60 mL of a solution containing 0.75 g of soluble starch R in 100 mL of water. Use the plate directly without heating. As the mobile phase use a mixture of 20 volumes of ammonia (~260 g/L) TS, 35 volumes of 2-propanol R and 55 volumes of ethyl acetate R. For the preparation of the test and reference solutions make up the following solvent mixture: to 5 volumes of ammonia (~260 g/L) TS add 70 volumes of methanol R and mix. For solution A dissolve 0.10 g of the test substance in the solvent mixture to produce 5 mL of concentrated solution, then dilute 1.0 mL of this solution with the same solvent mixture to produce 2.0 mL of diluted solution. For solution B dissolve 50 mg of levothyroxine sodium RS in the ammonia/methanol solvent mixture to produce 5 mL. For solution C dissolve 5 mg of liothyronine sodium RS in the ammonia/methanol solvent mixture to produce 25 mL of concentrated solution, then dilute 1.0 mL of this solution with the same solvent mixture to produce 2.0 mL of diluted solution. For solution D mix 1.0 mL of the concentrated solution A with 1.0 mL of the concentrated solution C. Apply separately to the plate 5 μL of each of diluted solution A, solution B, diluted solution C and solution D. After removing the plate from the chromatographic chamber allow it to dry in air, spray it with ferric chloride/ferricyanide/arsenite TS and examine the chromatogram in daylight. Any spot corresponding to liothyronine in the chromatogram obtained with solution A is not more intense than the spot in the chromatogram obtained with solution C. The test is not valid unless the chromatogram obtained with solution D shows two clearly separated spots.
Soluble halides. Shake 10 mg with 10 mL of water containing about 0.05 mL of nitric acid (~130 g/L) TS for 5 minutes and filter. Dilute the filtrate to 10 mL with water and add 0.15 mL of silver nitrate (40 g/L) TS; any opalescence produced is not more intense than that of a solution simultaneously prepared by adding 0.15 mL of silver nitrate (40 g/L) TS and 0.10 mL of hydrochloric acid (0.02 mol/L) VS to 10 mL of water (7 mg/g as chlorides).
Assay
Carry out the combustion as described under 2.4 Oxygen flask method using about 25 mg of the test substance, accurately weighed, and 10 mL of sodium hydroxide (10 g/L) TS as the absorbing liquid. When the process is complete proceed as described under the 4.2 Determination of iodine value. Each mL of sodium thiosulfate (0.05 mol/L) VS is equivalent to 1.665 mg of C15H10I4NNaO4.