Monographs: Pharmaceutical substances: Mefloquine hydrochloride (Mefloquini hydrochloridum)
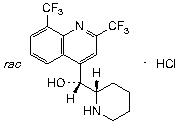
C17H16F6N2O,HCl
Relative molecular mass. 414.8
Chemical name. rac-(R)-[2,8-bis(trifluoromethyl)quinolin-4-yl][(2S)-piperidin-2-yl]methanol monohydrochloride; dl-erythro-α-2-piperidyl-2,8-bis(trifluoromethyl)-4-quinolinemethanol monohydrochloride; (R*,S*)-(±)-α-piperidin-2-yl-2,8-bis(trifluoromethyl)-4-quinolinemethanol monohydrochloride; CAS Reg. No. 51773-92-3.
Description. A white to slightly yellow, crystalline powder.
Solubility. Very slightly soluble in water; freely soluble in methanol R; soluble in ethanol (~750 g/L) TS; sparingly soluble in dichloromethane R.
Category. Antimalarial.
Storage. Mefloquine hydrochloride should be kept in a tightly closed container, protected from light.
Additional information. Mefloquine hydrochloride may exhibit polymorphism. It melts at about 260 °C, with decomposition.
Requirements
Mefloquine hydrochloride contains not less than 99.0% and not more than 101.0% of C17H16F6N2O,HCl, calculated with reference to the anhydrous substance.
Identity tests
- Either tests A and D or tests B, C and D may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from mefloquine hydrochloride RS or with the reference spectrum of mefloquine hydrochloride. If the spectra thus obtained are not concordant repeat the test using the residues obtained by separately dissolving the test substance and mefloquine hydrochloride RS in methanol R and evaporating to dryness. The infrared absorption spectrum is concordant with the spectrum obtained from mefloquine hydrochloride RS.
B. Carry out test B.1 or, where UV detection is not available, test B.2.
B.1 Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using silica gel R6 as the coating substance and a mixture of 70 volumes of toluene R, 30 volumes of ethanol R and 2 volumes of ammonia (~260 g/L) TS as the mobile phase. Apply separately to the plate 10 μL of each of the following two solutions in methanol R. For solution (A) use 10 mg of the test substance per mL. For solution (B) use 10 mg of mefloquine hydrochloride RS per mL. After removing the plate from the chromatographic chamber allow it to dry in a current of air and examine the chromatogram in ultraviolet light (254 nm).
The principal spot obtained with solution (A) corresponds in position, appearance and intensity to that obtained with solution (B).
B.2 Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using the conditions described above under test B.1 but using silica gel R5 as the coating substance. Stain the plate with iodine vapours. Examine the chromatogram in daylight.
The principal spot obtained with solution (A) corresponds in position, appearance and intensity to that obtained with solution (B).
C. The absorption spectrum (1.6) of a 50 μg/mL solution in methanol R, when observed between 250 nm and 290 nm, exhibits one maximum at about 283 nm.
D. A 50 mg/mL solution yields reaction B described under 2.1 General identification tests as characteristic of chlorides.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, procedure 3; determine the heavy metals content according to method A; not more than 20 μg/g.
Sulfated ash. (2.3) Not more than 1.0 mg/g.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, method A, using 250 mg of the substance; the water content is not more than 30 mg/g.
Related substances
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using a stainless steel column (25 cm x 4.6 mm) packed with particles of silica gel, the surface of which has been modified with chemically-bonded octadecylsilyl groups (5 μm).
As the mobile phase use a mixture of 22 volumes of methanol R, 38 volumes of acetonitrile R and 40 volumes of buffer pH 3.5 prepared as follows: dissolve 13.6 g potassium dihydrogen phosphate in about 900 mL of water R, adjust the pH to 3.5 by addition of phosphoric acid (~105 g/L) TS and dilute to 1000 mL.
Prepare the following solutions in the mobile phase. For solution (1) use about 2.2 mg of the test substance per mL. For solution (2) dilute a suitable volume of solution (1) to obtain a concentration equivalent to 4.4 μg of Mefloquine hydrochloride per mL. For solution (3) use about 0.22 mg of mefloquine hydrochloride RS and about 0.04 mg of sulfadoxine R per mL.
Operate with a flow rate of 1.5 mL per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of about 283 nm.
Inject 20 μL of solution (3). The test is not valid unless the resolution between the two principal peaks is at least 5.
Inject alternately 20 µL each of solutions (1) and (2). Record the chromatograms for about 10 times the retention time of mefloquine.
In the chromatogram obtained with solution (1) the following impurities, if present, are eluted at the following relative retention with reference to mefloquine (retention time about 3.9 minutes): impurity A about 0.9; impurity C about 3.6; and impurity B about 7.4.
In the chromatogram obtained with solution (1):
- the area of any peak corresponding to impurity A is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
- the area of any other peak, apart from the principal peak, is not greater than 0.5 times the area of the peak in the chromatogram obtained with solution (2) (0.1%);
- the sum of the areas of all peaks, other than the peak due to mefloquine, is not greater than 2.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%). Disregard any peak with an area less than 0.25 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).
Assay
Dissolve 0.350 g, accurately weighed, in 15 mL of anhydrous formic acid R and add 40 mL of acetic anhydride R. Titrate with perchloric acid (0.1 mol/L) VS, determining the end-point potentiometrically. Each mL of perchloric acid (0.1 mol/L) VS is equivalent to 41.48 mg of C17H17ClF6N2O.
Impurities

A. rac-(R)-[2,8-bis(trifluoromethyl)quinolin-4-yl][(2R)-piperidin-2-yl]methanol (threo-mefloquine)

B. [2,8-bis(trifluoromethyl)quinolin-4-yl](pyridin-2-yl)methanone

C. rac-(R)-[2,8-bis(trifluoromethyl)quinolin-4-yl](pyridin-2-yl)methanol