Monographs: Pharmaceutical substances: Quinine sulfate (Quinini sulfas)
Molecular formula. (C20H24N2O2)2,H2SO4,2H2O
Relative molecular mass. 783.0
Graphic formula.
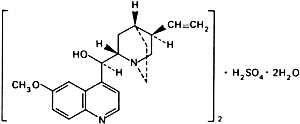
Chemical name. (8α,9R)-6'-Methoxycinchonan-9-ol sulfate (2:1) (salt) dihydrate; (8α,9R)-9-hydroxy-6'-methoxycinchonan sulfate (2:1) (salt) dihydrate; CAS Reg. No. 6591-63-5.
Description. Colourless, needle-like crystals; odourless.
Solubility. Slightly soluble in water, ethanol (~750 g/l) TS and ether R.
Category. Antimalarial.
Storage. Quinine sulfate should be kept in a well-closed container, protected from light.
Additional information. Quinine sulfate has a very bitter taste.
Requirements
Definition. Quinine sulfate contains not less than 99.0% and not more than 101.0% of total alkaloids, calculated as (C20H24N2O2)2,H2SO4 and with reference to the dried substance.
Identity tests
A. Dissolve 5 mg in 10 mL of water and add 1 drop of sulfuric acid (~100 g/l) TS; a strong blue fluorescence is produced.
B. To 5 mL of a 1 mg/mL solution add 2-3 drops of bromine TS1 and 5 drops of ammonia (~100 g/l) TS; an emerald-green colour is produced.
C. A 20 mg/mL solution yields reaction A described under 2.1 General identification tests as characteristic of sulfates.
Specific optical rotation (1.4). Use a 30 mg/mL solution in sulfuric acid (~100 g/l) TS and calculate with reference to the dried substance;  = -240° to -250°.
= -240° to -250°.
Clarity and colour of solution. Dissolve 20 mg in 5 mL of hydrochloric acid (0.1 mol/L) VS and add sufficient water to produce 10 mL. This solution is clear and not more intensely coloured than standard colour solution Yw2 when compared as described under 1.11.1 Colour of liquids.
Sulfated ash (2.3). Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not less than 30 mg/g and not more than 50 mg/g.
pH value (1.13). pH of a 10 mg/mL suspension in carbon-dioxide-free water R, 5.7-6.6.
Related cinchona alkaloids.
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a stainless steel column (15 cm x 4.6 mm) packed with particles of silica gel, the surface of which has been modified with chemically bonded octadecylsilyl groups (5 µm).
As the mobile phase, use a solution prepared as follows. Dissolve 6.8 g of potassium dihydrogen phosphate R and 3.0 g of hexylamine R in 900 mL of water R, adjust to pH 3.0 with phosphoric acid (~1440 g/l) TS and dilute to 1000 mL with water R. Mix 920 mL of this solution with 80 mL of acetonitrile R.
Prepare the following solutions in the solvent consisting of 80 volumes of water R, 20 volumes of acetonitrile R and 0.1 volume of phosphoric acid (~1440 g/l) TS. For solution (1) dissolve a quantity of the test substance to obtain a concentration of about 3 mg per mL. For solution (2) dissolve about 30 mg of quinine sulfate RS in 10 mL of the solvent. For solution (3) dissolve about 15 mg of quinidine sulfate RS in 5 mL of solution (2).
Operate with a flow rate of 1.3 mL per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of about 316 nm.
Inject separately 10 μl each of solution (1), (2) and (3) in the chromatographic system and record the chromatograms for 2.5 times the retention time of the quinine (principal) peak in solution (2).
In the chromatogram obtained with solution (3), the following peaks are eluted at the following relative retention with reference to quinine (retention time about 10 minutes): quinidine about 0.8; dihydroquinidine about 1.2; dihydroquinine about 1.5. The test is not valid unless the resolution between the peaks due to quinidine and quinine and that between the peaks due to quinine and dihydroquinidine is at least 1.5. The chromatograms obtained with solutions (1), (2) and (3) may also show a peak due to cinchonidine eluting at a relative retention of about 0.6 with reference to quinine.
Calculate the percentage content of the related substances in the chromatogram obtained with solution (1) by normalisation. The content of dihydroquinine is not more than 10%, the content of cinchonidine not more then 5% and the content of any other related substance not more than 2.5%. The sum of the related substances is not more than 15%. Disregard any related substance of content less than 0.1%.
Assay. Dissolve about 0.20 g, accurately weighed, in 30 mL of glacial acetic acid R1, add 20 mL of acetic anhydride R, and titrate with perchloric acid (0.1 mol/l) VS as described under 2.6 Non-aqueous titration. Method A. Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 24.90 mg of (C20H24N2O2)2,H2SO4.
Impurities
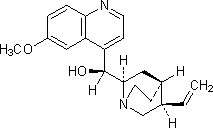
A. (S)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]oct-2-yl](6-methoxyquinolin-4-yl)methanol (quinidine),
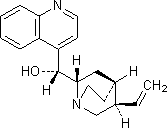
B. (R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]oct-2-yl](quinolin-4-yl)methanol (cinchonidine),
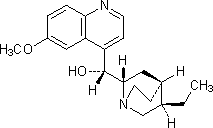
C. (R)-[(2S,4S,5R)-5-ethyl-1-azabicyclo[2.2.2]oct-2-yl](6-methoxyquinolin-4-yl)methanol (dihydroquinine).