Monographs: Pharmaceutical substances: Tetracaine hydrochloride (Tetracaini hydrochloridum)
Molecular formula. C15H24N2O2,HCl
Relative molecular mass. 300.8
Graphic formula.
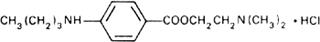
Chemical name. 2-(Dimethylamino)ethyl p-(butylamino)benzoate monohydrochloride; 2-(dimethylamino)ethyl 4-(butylamino)benzoate monohydrochloride; CAS Reg. No. 136-47-0.
Other names. Amethocaine hydrochloride; dicainum.
Description. A white, crystalline powder; odourless.
Solubility. Soluble in about 8 parts of water; soluble in ethanol (~750 g/l) TS; practically insoluble in ether R.
Category. Local anaesthetic.
Storage. Tetracaine hydrochloride should be kept in a tightly closed container, protected from light.
Additional information. Tetracaine hydrochloride is hygroscopic; it has a slightly bitter taste and causes local numbness after being placed on the tongue. Even in the absence of light, it is gradually degraded on exposure to a humid atmosphere, the decomposition being faster at higher temperatures.
Tetracaine hydrochloride melts at about 148 °C or may exist in either of the two polymorphic forms, one of which melts at 134 °C and the other at 139 °C. Mixtures of the forms melt within the range 134-147°C.
Requirements
Definition. Tetracaine hydrochloride contains not less than 98.0% and not more than 101.0% of C15H24N2O2,HCl, calculated with reference to the dried substance.
Identity tests
A. Dissolve 0.2 g in 10 mL of water and add 1 mL of ammonium thiocyanate (75 g/l) TS. Collect the precipitate on a filter, recrystallize from water, and dry it at 80°C for 2 hours; melting temperature, about 131 °C.
B. A 20 mg/mL solution yields reaction A described under 2.1 General identification tests as characteristic of chlorides.
Clarity and colour of solution. A solution of 0.20 g in 10 mL of carbon-dioxide-free water R is clear and colourless.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 10 mg/g.
pH value. pH of a 10 mg/mL solution in carbon-dioxide-free water R, 4.5-6.0.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R4 as the coating substance and a mixture of 80 volumes of dibutyl ether R, 16 volumes of hexane R, and 4 volumes of glacial acetic acid R as the mobile phase. Place the plate in the chromatographic chamber, dipping it about 5 mm into the liquid. After the solvent has reached a height of about 12 cm, remove the plate from the chromatographic chamber and dry it for a few minutes in a current of warm air. Allow it to cool and apply separately to the plate 5 μl of each of 2 solutions containing (A) 0.10 g of the test substance per mL and (B) 0.050 mg of 4-aminobenzoic acid R per mL. Allow the solvent front to ascend 10 cm above the line of application. After removing the plate from the chromatographic chamber, dry it at 105°C for 10 minutes and examine the chromatogram in ultraviolet light (254 nm). Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B. The principal spot remains on the baseline.
Assay. Carry out the assay as described under 2.7 Nitrite titration, using about 0.5 g, accurately weighed, dissolved in a mixture of 50 mL of water and 5 mL of hydrochloric acid (~420 g/l) TS, and titrate with sodium nitrite (0.1 mol/l) VS. Each mL of sodium nitrite (0.1 mol/l) VS is equivalent to 30.08 mg of C15H24N2O2,HCl.
Additional requirements for Tetracaine hydrochloride for sterile use
Complies with 3.2 Test for sterility.