Monographs: Dosage forms: Specific monographs: Dolutegravir dispersible tablets (dolutegraviri compressi dispersibili)
Category. Antiretroviral (integrase strand-transfer inhibitor).
Storage. Dolutegravir dispersible tablets should be kept in a well-closed container.
Labelling. The designation of the container should state that the active ingredient is the sodium salt and the quantity should be indicated in terms of the equivalent amount of dolutegravir.
Additional information. Strength in the 19th invitation to manufacturers and suppliers of medicinal products for HIV infections and related diseases to submit an expression of interest (EOI) for product evaluation to the WHO Prequalification Team - Medicines: 5 mg or 10 mg per dispersible tablet.
Requirements
Complies with the monograph for Tablets.
Definition. Dolutegravir dispersible tablets contain Dolutegravir sodium in a suitable dispersible basis. They contain not less than 90.0% and not more than 110.0% of the amount of dolutegravir (C20H19F2N3O5) stated on the label.
Identity tests
-
Any two of tests A, B or C may be applied.
-
Carry out the test as described under 1.14.1 Chromatography, High-Performance Liquid Chromatography using the conditions and solutions given under "Assay", Method A. The retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to the retention time of the peak due to dolutegravir in the chromatogram obtained with solution (2).
-
To a quantity of the powdered dispersible tablets, nominally equivalent to 10 mg of dolutegravir, add 40 mL methanol R, sonicate for five minutes, allow to cool to room temperature, dilute to 50 mL and filter. Dilute 1 mL of the filtrate to 20 mL with methanol R. The absorption spectrum (1.6) of the resulting solution, when observed between 220 nm and 400 nm, exhibits a maximum at about 258 nm.
Alternatively, in combination with identity test A, where a diode array detector is available, record the UV spectra of the principal peaks in the chromatograms with a diode array detector in the range of 220 nm to 400 nm. The UV spectrum of the principal peak in the chromatogram obtained with solution (1) correspond to the spectrum of the peak due to dolutegravir in the chromatogram obtained with solution (2).
-
Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R6, or similar, as the coating substance and a mixture of 72 volumes of ethyl acetate R, 14 volumes of water R and 14 volumes of glacial acetic acid R as the mobile phase. Prepare, as a solvent solution, a mixture of 96 volumes of methanol R and 4 volumes of glacial acetic acid R. Apply separately to the plate 5 μL of each of the following two solutions. For solution (A), shake a quantity of the powdered dispersible tablets, nominally equivalent to 10 mg of dolutegravir with 10 mL of the solvent solution and filter. For solution (B), use a solution containing 1.1 mg of dolutegravir sodium RS per mL of solvent solution. After removing the plate from the chromatographic chamber, allow it to dry in air or in a current of cool air. Examine the chromatogram in ultraviolet light (254 nm).
The principal spot obtained with solution (A) corresponds in position, appearance and intensity with that obtained with solution (B).
After drying, spray the plate with basic potassium permanganate (5 g/L) TS. Examine the chromatogram in daylight. The principal spot obtained with solution (A) corresponds in position, appearance and intensity with that obtained with solution (B).
Disintegration. Carry out the test as described under 5.3 Disintegration test for tablets and capsules, but using water R at 15 to 25 °C. The dispersible tablets disintegrate within 3 minutes.
Dissolution. Carry out the test as described under 5.5 Dissolution test for oral dosage forms using as the dissolution medium 900 mL of a solution prepared by dissolving 2.5 g of sodium dodecyl sulfate R in 1000 mL dissolution buffer pH 6.8 TS. Rotate the paddle at 50 revolutions per minute. At 30 minutes, withdraw a sample of 10 mL of the medium through an in-line filter. Allow the filtered solution to cool down to room temperature and dilute 5.0 mL of the filtered solution to 20.0 mL with dissolution medium. Deaeration of the solvent may be necessary to avoid the formation of microbubbles that may interfere with the determination of the absorbance.
Measure the absorbance of the resulting solution as described under 1.6 Spectrophotometry in the visible and ultraviolet regions in a cuvette with an optical pathlength of 10 mm at the maximum at about 258 nm, using the dissolution medium as the blank.
For each of the dispersible tablets tested, calculate the amount of dolutegravir (C20H19F2N3O5) in the medium using the absorptivity value of 55.8 for dolutegravir sodium (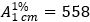 ). Each mg of dolutegravir sodium is equivalent to 0.950 mg of dolutegravir.
). Each mg of dolutegravir sodium is equivalent to 0.950 mg of dolutegravir.
Evaluate the results as described under 5.5 Dissolution test for oral dosage forms, Acceptance criteria. The amount of dolutegravir in solution for each tablet is not less than 80% (Q) of the amount declared on the label.
Related substances. Perform the test in subdued light and without any prolonged interruptions, using low-actinic glassware.
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a stainless steel column (25 cm x 4.6 mm) packed with particles of silica gel, the surface of which has been modified with chemically-bonded octadecylsilyl and pentafluorophenyl groups (5 µm).
Use the following conditions for gradient elution:
- Mobile phase A: 0.186 g disodium edetate R in 1000 mL water R adjusted to pH 2.0 with phosphoric acid (~20 g/L) TS;
- Mobile phase B: 90 volumes of methanol R and 10 volumes of tetrahydrofuran R.
|
Time (minutes) |
Mobile phase A |
Mobile phase B |
Comments |
|
0-2 |
60 |
40 |
Isocratic |
|
2-32 |
60 to 50 |
40 to 50 |
Linear gradient |
|
32-56 |
50 to 20 |
50 to 80 |
Linear gradient |
|
56-62 |
20 |
80 |
Isocratic |
|
62-63 |
20 to 60 |
80 to 40 |
Return to initial composition |
|
63-70 |
60 |
40 |
Re-equilibration |
Operate at a flow rate of 1.0 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of 258 nm. Maintain the column temperature at 45 °C.
Prepare the following solutions using as the diluent a mixture of 60 volumes of water R and 40 volumes of acetonitrile R.
For solution (1), transfer a quantity of the powdered dispersible tablets, nominally equivalent to 70.0 mg dolutegravir, to a 100 mL volumetric flask. Add about 70 mL diluent and sonicate for five minutes, cool to room temperature, dilute to volume and filter. For solution (2), dilute 1.0 mL of solution (1) to 100.0 mL. Dilute 10.0 mL of this solution to 50.0 mL. For solution (3), prepare a solution containing 0.04 mg of dolutegravir impurity D RS per mL of acetonitrile R. For solution (4), dissolve 5 mg of dolutegravir sodium RS in 1 mL acetonitrile R. Add 4.5 mL water R and 4.5 mL hydrochloric acid (~ 420 g/L) TS and boil the solution under reflux for 1 hour. Cool the solution to room temperature and dilute 1 mL of it to 10 mL with a mixture of 6 volumes of water R and 4 volumes of acetonitrile R. For solution (5), mix 1 mL of solution (3) with 1 mL of solution (4).
Inject 10 µL of solutions (1), (2), (3), (4) and (5).
Use the chromatogram obtained with solution (3) and the chromatogram supplied with dolutegravir impurity D RS to identify the peak due to the impurity D. Use the chromatogram obtained with solution (4) to identify the peak due to impurity H (the chromatogram usually shows two principal peaks: the peak due to dolutegravir and the peak due to impurity H).
The impurities, if present, are eluted at the following relative retentions with reference to dolutegravir (retention time about 30 minutes): impurity C about 0.66; impurity F about 0.70; impurity D about 0.74; impurity H about 0.78; impurity E about 0.89; impurity J about 1.75; impurity K about 1.77; impurity L about 2.10.
The test is not valid unless, in the chromatogram obtained with solution (5), the resolution between the peaks due to impurity D and impurity H is at least 1.5. Also, the test is not valid unless, in the chromatogram obtained with solution (2), the peak due to dolutegravir is obtained with a signal-to-noise ratio of at least 20.
In the chromatogram obtained with solution (1):
-
the area of any impurity peak is not greater than the area of the peak due to dolutegravir in the chromatogram obtained with solution (2) (0.2%).
-
The sum of the areas of all impurity peaks is not greater than 5 times the area of the peak due to dolutegravir in the chromatogram obtained with solution (2) (1.0%). Disregard any peak with an area less than 0.5 times the area of the peak due to dolutegravir in the chromatogram obtained with solution (2) (0.1%).
Assay
-
For 5 mg tablets, either methods A or B may be applied.
-
For 10 mg tablets, calculate the percentage content of the declared dolutegravir quantity in the total weight of the dispersible tablets. For dispersible tablets, where the declared quantity of dolutegravir is 5% or less of the total weight of the dispersible tablets, either methods A or B may be applied. For dispersible tablets where the declared quantity of dolutegravir is more than 5%, apply method A.
-
Perform the test in subdued light and without any prolonged interruptions, using low-actinic glassware. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a stainless steel column (25 cm x 4.6 mm) packed with particles of silica gel, the surface of which has been modified with octadecylsilyl and pentafluorophenyl groups (5 µm).
Use the following mobile phase: dissolve 0.186 g of disodium edetate R in 1000 mL water R and adjust to pH 3.0 with phosphoric acid (~20 g/L) TS. Mix 420 volumes of this solution with 580 volumes of methanol R.
Operate at a flow rate of 1.0 mL/minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of 258 nm. Maintain the column at a temperature of 40 °C.
Prepare the following solutions using as the diluent a mixture of 60 volumes of water R and 40 volumes of acetonitrile R.
For solution (1), weigh and powder 20 dispersible tablets. Transfer a quantity of the powdered tablets, nominally equivalent to 100.0 mg of dolutegravir, to a 100 mL volumetric flask. Add about 70 mL of diluent and sonicate for five minutes, cool to room temperature and make up to volume with diluent. Filter and dilute 5.0 mL of the filtrate to 100.0 mL. For solution (2), dissolve 53.0 mg of dolutegravir sodium RS and dilute to 50.0 mL. Dilute 5.0 mL of this solution to 100.0 mL.
Inject 20 µL each of solutions (1) and (2). Record the chromatograms for about 20 minutes.
Measure the areas of the peaks corresponding to dolutegravir obtained in the chromatograms of solutions (1) and (2) and calculate the percentage content of dolutegravir (C20H18F2N3O5) in the dispersible tablets using the declared content of C20H18F2N3NaO5 in dolutegravir sodium RS. Each mg of dolutegravir sodium is equivalent to 0.950 mg of dolutegravir.
-
Use the average of 10 individual results obtained in the test for "Uniformity of content".
Uniformity of content. 5 mg dispersible tablets comply with the test for 5.1 Uniformity of content for single-dose preparations using the method of analysis described below; 10 mg dispersible tablets comply with the test in case the declared quantity of dolutegravir is 5% or less of the total weight of the dispersible tablet.
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using the chromatographic conditions as described under "Assay", Method A
Prepare the following solutions using as the diluent a mixture of 60 volumes of water R and 40 volumes of acetonitrile R.
For solution (1), transfer one dispersible tablet to a 20 mL volumetric flask, add about 14 mL of diluent and sonicate for five minutes, cool to room temperature and make up to volume with the diluent and filter. For 5 mg tablets, dilute 20.0 mL of the filtrate to 100.0 mL. For 10 mg tablets, dilute 10.0 mL of the filtrate to 100.0 mL. For solution (2), dissolve 53.0 mg of dolutegravir sodium RS and dilute to 50.0 mL. Dilute 5.0 mL of this solution to 100.0 mL.
Inject 20 µL each of solutions (1) and (2). Record the chromatograms for about 20 minutes.
Measure the areas of the peaks corresponding to dolutegravir obtained in the chromatograms of solutions (1) and (2) and calculate the percentage content of dolutegravir (C20H18F2N3O5) per dispersible tablet using the declared content of C20H18F2N3NaO5 in dolutegravir sodium RS. Each mg of dolutegravir sodium is equivalent to 0.950 mg of dolutegravir.
Impurities. The impurities limited by the requirements of this monograph include those listed in the monograph on Dolutegravir sodium, excluding impurities A, B and G.