Monographs: Dosage forms: Specific monographs: Levofloxacin tablets (Levofloxacini compressi)
Category. Antibacterial, antibuberculosis.
Storage. Levofloxacin tablets should be kept in a well closed container, protected from light.
Labelling. The designation of the container of Levofloxacin tablets should state that the active ingredient is Levofloxacin hemihydrate and the quantity should be indicated in terms of the equivalent amount of levofloxacin.
Additional information. Strengths in the current WHO Model list of essential medicines (EML): 250 mg, 500 and 750 mg. Strengths in the current WHO EML for children: 250 mg and 500 mg.
Requirements
Comply with the monograph for Tablets.
Definition. Levofloxacin tablets contain Levofloxacin hemihydrate. They contain not less than 90.0% and not more than 110.0% of the amount of levofloxacin (C18H20FN3O4) stated on the label.
Identity test
Either test A or tests B and C may be applied.
-
To a quantity of the powdered tablets, nominally equivalent to 100 mg of levofloxacin, add 10 mL of acetonitrile R, shake, filter and evaporate to dryness. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from levofloxacin RS or with the reference spectrum of levofloxacin hemihydrate.
If the spectra thus obtained are not concordant, repeat the test using the residues obtained by separately dissolving the test substance and levofloxacin RS in a small amount of acetonitrile R and evaporating to dryness. The infrared absorption spectrum is concordant with the spectrum obtained from levofloxacin RS.
-
Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R5 as the coating substance and a mixture of 10 volumes of dichloromethane R, 5 volumes of methanol R and 1 volume of ammonia (~10 g/L) TS as the mobile phase. Apply separately to the plate 5 ml of each of the following two solutions in a mixture of 1 volume of methanol R and 4 volumes of dichloromethane R. For solution (A), shake a quantity of the powdered tablets, nominally equivalent to 25 mg of levofloxacin, with 5 mL, filter and use the clear filtrate. For solution (B), use a solution containing 5 mg of levofloxacin RS per mL. After removing the plate from the chromatographic chamber, allow it to dry exhaustively in air or in a current of cool air. Examine the chromatogram in ultraviolet light (366 nm).
The principal spot in the chromatogram obtained with solution (A) corresponds in position, appearance and intensity with the spot due to levofloxacin in the chromatogram obtained with solution (B).
- Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using the conditions given under "Assay". The retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to the retention time of the peak due to levofloxacin in the chromatogram obtained with solution (2).
Dissolution. Carry out the test as described under 5.5 Dissolution test for oral dosage forms, using as the dissolution medium 900 mL of hydrochloric acid (~3.65 g/L) TS and rotating the basket at 100 revolutions per minute. At 30 minutes, withdraw a sample of about 10 mL of the medium through an in-line filter. Allow the filtered sample to cool to room temperature and dilute 1.0 mL to 100.0 mL with hydrochloric acid (~3.65 g/L) TS.
Measure the absorbance (1.6) of a 1 cm layer of the sample at about 294 nm.
For each of the tablets, calculate the total amount of levofloxacin (C18H20FN3O4), in the medium, using an absorptivity value of 90.66 (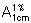 = 906.6) for levofloxacin. Evaluate the results as described under 5.5 Dissolution test for oral dosage forms, Acceptance criteria. The amount of levofloxacin released is not less than 80% (Q) of the amount declared on the label.
= 906.6) for levofloxacin. Evaluate the results as described under 5.5 Dissolution test for oral dosage forms, Acceptance criteria. The amount of levofloxacin released is not less than 80% (Q) of the amount declared on the label.
Related substances. Prepare fresh solutions, protected from light, and perform the test without delay.
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using the conditions given under "Assay".
Prepare the following solutions in mobile phase. For solution (1), transfer a quantity of the powdered tablets, nominally equivalent to 250.0 mg of levofloxacin, into a 250 mL volumetric flask, add about 180 mL, sonicate for 5 minutes, dilute to volume, mix and filter. For solution (2), dilute 1.0 mL of solution (1) to 100.0 mL. For solution (3), dilute 1.0 mL of solution (2) to 10.0 mL. For solution (4), use a solution containing 1.0 mg of levofloxacin for system suitability RS (containing levofloxacin and the impurities A, B and G) per mL.
Inject 25 μL of solutions (1), (2), (3) and (4). Record the chromatogram for about three times the retention time of levofloxacin.
Use the chromatogram supplied with levofloxacin for system suitability RS and the chromatogram obtained with solution (3) to identify the peaks due to impurities A, B and G. The impurities, if present, are eluted at the following relative retentions with reference to levofloxacin (retention time about 20 minutes): impurity E about 0.38, impurity B about 0.47, impurity G about 0.52, impurity C about 0.63, impurity D about 0.73, impurity A about 1.22, impurity I about 1.69.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to impurity B and impurity G is at least 1.5.
In the chromatogram obtained with solution (1):
-
the area of any peak corresponding to impurity B, when multiplied by a correction factor of 1.3, is not greater than seven times the area of the peak due to levofloxacin in the chromatogram obtained with solution (3) (0.7%);
-
the area of any peak corresponding to impurity C, when multiplied by a correction factor of 1.47, is not greater than seven times the area of the peak due to levofloxacin in the chromatogram obtained with solution (3) (0.7%);
-
the area of any peak corresponding to impurity E, when multiplied by a correction factor of 1.67, is not greater than three times the area of the peak due to levofloxacin in the chromatogram obtained with solution (3) (0.3%);
-
the area of any peak corresponding to impurity G, when multiplied by a correction factor of 1.20, is not greater than three times the area of the peak due to levofloxacin in the chromatogram obtained with solution (3) (0.3%);
-
the area of any other impurity peak, other than any peak due to impurity A, is not greater than twice the area of the peak due to levofloxacin in the chromatogram obtained with solution (3) (0.2%).
-
The sum of the corrected areas of any peak corresponding to impurities B, C, E and G and the areas of all other impurity peaks, other than any peak due to impurity A, is not greater than the area of the peak due to levofloxacin in the chromatogram obtained with solution (2) (1%). Disregard any peak with an area less the area of the peak due to levofloxacin in the chromatogram obtained with solution (3) (0.1%).
Assay. Prepare fresh solutions, protected from light and perform the test without delay.
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using a stainless steel column (25 cm x 4.6 mm), packed with end-capped and base-deactivated particles of silica gel, the surface of which has been modified with chemically-bonded octadecylsilyl groups (5 μm).
Prepare the following buffer solution. Dissolve 1.25 g of copper sulfate pentahydrate R, 1.3 g of isoleucine R and 8.5 g of ammonium acetate R in water R and dilute to 1000 mL with the same solvent.
As the mobile phase, use a mixture of methanol R and buffer solution (30:70 v/v). Operate with a flow rate of 0.8 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of 360 nm. Maintain the column temperature at 45 °C.
Weigh and powder 20 tablets. For solution (1), transfer a quantity of the powdered tablets, nominally equivalent to 50.0 mg of levofloxacin, into a 250 mL volumetric flask, add about 180 mL of the mobile phase, shake for 30 minutes, dilute to volume, mix and filter. For solution (2), dissolve 20.0 mg of levofloxacin RS in mobile phase and dilute to 100.0 mL using the same solvent.
Inject alternately 10 μL each of solutions (1) and (2) and record the chromatograms for about two times the retention time of levofloxacin (about 20 minutes).
Measure the areas of the peaks corresponding to levofloxacin obtained in the chromatograms of solutions (1) and (2) and calculate the percentage content of levofloxacin (C18H20FN3O4) in the tablets, using the declared content of C18H20FN3O4 in levofloxacin RS.
Impurities. The impurities limited by the requirements of this monograph include those listed in the monograph for Levofloxacin hemihydrate.