Monographs: Pharmaceutical substances: Saccharin sodium (Saccharinum natricum)
Saccharin sodium, anhydrous
Saccharin sodium, dihydrate
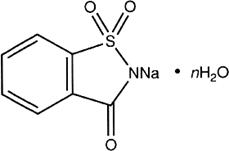
n = 0 (anhydrous)
n = 2 (dihydrate)
C7H4NNaO3S (anhydrous)
C7H4NNaO3S,2H2O (dihydrate)
Relative molecular mass. 205.2 (anhydrous); 241.2 (dihydrate).
Chemical name. 1,2-Benzisothiazolin-3-one 1,1-dioxide, sodium salt; 1,2-benzisothiazol-3(2H)-one 1,1-dioxide, sodium salt; CAS Reg. No. 128-44-9 (anhydrous).
1,2-Benzisothiazolin-3-one 1,1-dioxide, sodium salt, dihydrate; 1,2-benzisothiazol-3(2H)-one 1,1-dioxide, sodium salt, dihydrate; CAS Reg. No. 6155-57-3 (dihydrate).
Other name. Saccharimidum natricum.
Description. Colourless crystals or a white, crystalline powder; odourless or almost odourless.
Solubility. Freely soluble in water; sparingly soluble in ethanol (~750 g/l) TS; practically insoluble in ether R.
Category. Sweetening agent.
Storage. Saccharin sodium should be kept in a well-closed container.
Additional information. Saccharin sodium effloresces slowly in air and loses about half of its content of water of crystallization. It has a very sweet taste, even in very dilute solutions.
Requirements
Saccharin sodium contains not less than 98.0% and not more than the equivalent of 101.0% of C7H4NNaO3S, calculated with reference to the anhydrous substance.
Identity tests
A. To 20 mg add 0.04 g of resorcinol R and 0.5 mL of sulfuric acid (~1760 g/l) TS, and heat gently until a dark green colour is observed. Allow to cool and add 10 mL of water and 10 mL of sodium hydroxide (~80 g/l) TS; a fluorescent green solution is produced.
B. Ignite 1 g and proceed with the residue as follows:
- Dissolve half of the residue in acetic acid (~60 g/l) TS. When tested for sodium as described under 2.1 General identification tests it yields reaction B.
- Dissolve the remaining residue in hydrochloric acid (~70 g/l) TS. It yields reaction A described under 2.1 General identification tests as characteristic of sulfates.
Arsenic. Transfer 3.3 g to a crucible containing 3.3 g of anhydrous sodium carbonate R. Moisten with a small quantity of water, evaporate to dryness on a water-bath, and ignite to 550 °C until all black particles have disappeared. Cool, dissolve the residue in 5 mL of hydrochloric acid (~250 g/l) AsTS, and proceed as described under 2.2.5 Limit test for arsenic; the arsenic content is not more than 3 μg/g.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to Method A; not more than 20 μg/g.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A, using 1 g of Saccharin sodium dihydrate; the water content is not more than 150 mg/g.
Free acid or alkali. Dissolve 1 g in 10 mL of carbon-dioxide-free water R, add 5 mL of sulfuric acid (0.005 mol/l) VS, boil, cool, and titrate with sodium hydroxide (0.01 mol/l) VS using phenolphthalein/ethanol TS as indicator; 4.5-5.5 mL are required to obtain a pink colour.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance and a mixture of 100 volumes of chloroform R, 50 volumes of methanol R, and 10 volumes of ammonia (~260 g/l) TS as the mobile phase. Apply separately to the plate 5 μl of each of the following four solutions: For solution (A) dissolve 2.6 g of Saccharin sodium in 10 mL of sodium hydrogen carbonate (100 g/l) TS, add 12.5 g of diatomaceous support R as a filter-aid, and mix well. Transfer to a chromatographic tube, 250 mm in length and with a diameter of 25 mm, fitted at the lower end with a sintered-glass disc and a stopcock. Pack the contents of the tube by tapping on a padded surface and tamping firmly from the top. Elute with dichloromethane R at a rate of 50 mL in 30 minutes. Evaporate the eluate to dryness and dissolve the residue in 4 mL of acetone R. For solutions (B) dissolve 50μg of toluene-2-sulfonamide RS per mL of acetone R, (C) 5mg of Saccharin sodium per mL of methanol R, and (D) 50 μg of 4-sulfamoylbenzoic acid R per mL of acetone R. After removing the plate from the chromatographic chamber, dry in a current of warm air, heat at 105 °C for 5 minutes, and spray the hot plate with sodium hypochlorite TS1. Dry in a current of cold air until a sprayed area of the plate below the line of application gives at most a faint blue colour with 0.05 mL of a solution of 5 mg of potassium iodide R in 1 mL of starch TS containing 1% glacial acetic acid R. Avoid prolonged exposure to cold air. Spray the plate again with the same mixture. Examine the chromatogram in daylight.
Any spot obtained with solution A corresponding to toluene-2-sulfonamide is not more intense than that obtained with solution B. Any spot obtained with solution C corresponding to 4-sulfamoylbenzoic acid is not more intense than that obtained with solution D.
Assay. Dissolve about 0.3 g, accurately weighed, in 30 mL of glacial acetic acid R1, and titrate with perchloric acid (0.1 mol/l) VS as described under 2.6 Non-aqueous titration, Method A.
Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 20.52 mg of C7H4NNaO3S.